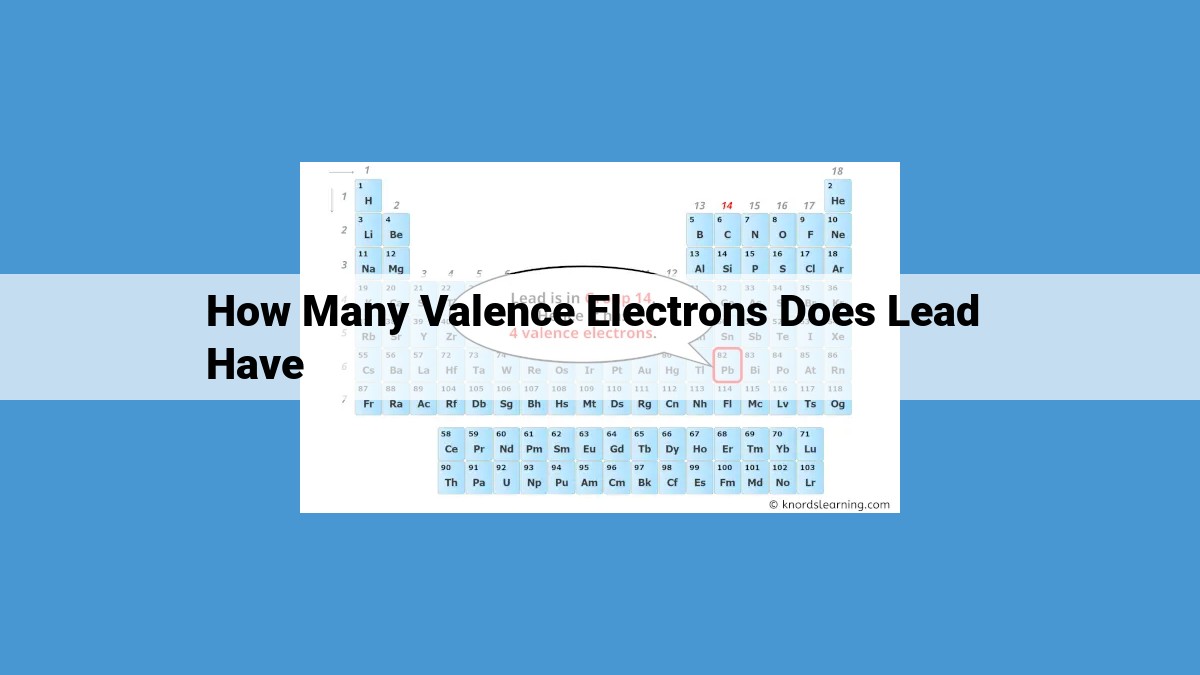
Lead (Pb), with an atomic number of 82, has 4 valence electrons. Valence electrons are those occupying the outermost shell of an atom and play a crucial role in determining an element’s chemical bonding behavior. Lead’s electron configuration is [Xe] 4f¹⁴ 5d¹⁰ 6s², indicating 2 valence electrons in the 6s orbital and 2 in the 5d orbital. These valence electrons enable lead to form covalent or ionic bonds with other elements, influencing its chemical properties and the formation of various compounds.
- Explain the purpose of the blog post: To determine the number of valence electrons in lead.
Determining the Valence Electrons of Lead: A Journey into Chemical Bonding
Imagine a world where atoms dance and interact, forming the very building blocks of the universe. At the heart of these atomic interactions lies a crucial concept: valence electrons. These electrons hold the key to understanding how elements bond together, creating the countless materials that shape our world.
One such element that demonstrates the fascinating role of valence electrons is lead. This enigmatic metal has been used for centuries in various applications, from ancient plumbing to modern batteries. But what makes lead so intriguing is its unique electronic configuration.
Unveiling the Mystery of Valence Electrons
To grasp the significance of valence electrons, we must first understand their nature. Valence electrons are the outermost electrons of an atom, the ones that dictate its chemical behavior. They determine an element’s ability to form bonds, whether with itself or with other elements.
The atomic number of an element directly correlates with the number of valence electrons it possesses. Lead, with an atomic number of 82, boasts four valence electrons. Imagine these electrons as lead’s “social butterflies,” eagerly seeking opportunities to bond and form connections.
Lead’s Electronic Make-up
Lead’s electronic configuration, a roadmap to its atomic structure, reveals its valence electron behavior. Written as 1s22s22p63s23p64s23d104p65s25p65d106s26p2, this notation guides us through the energy levels and orbitals occupied by lead’s electrons.
Crucially, the outermost energy level, labeled 6s26p2, houses two valence electrons. These two electrons, like lone adventurers, seek chemical bonds to stabilize lead’s electronic structure.
Impact on Chemical Bonding and Material Properties
The number of valence electrons plays a vital role in lead’s chemical reactivity. With two valence electrons, lead exhibits a valency of two. This means it can form two covalent bonds with other elements or share its valence electrons to form ionic bonds.
Lead’s valence electrons are responsible for its ability to form a wide range of compounds, from lead oxide to lead sulfide. These compounds find applications in diverse fields such as batteries, pigments, and radiation shielding.
In summary, lead possesses four valence electrons, a characteristic that stems from its atomic number and electronic configuration. These valence electrons govern lead’s chemical behavior, enabling it to form bonds and participate in a multitude of chemical reactions. Understanding valence electrons is fundamental to comprehending the intricate dance of atoms and the properties of the materials they form.
Understanding Valence Electrons: The Key to Chemical Bonding
In the realm of chemistry, the dance of electrons plays a crucial role in shaping the properties of elements and their interactions. Among these electrons, valence electrons hold a special significance, influencing the very nature of chemical bonds.
Defining Valence Electrons
Valence electrons are the electrons that occupy the outermost energy level of an atom, residing at the energy threshold between bonding and non-bonding states. These electrons are the active participants in chemical reactions, eagerly seeking to form bonds with other atoms to achieve a stable configuration.
Connecting Atomic Number to Valence Electrons
The number of valence electrons in an element is directly linked to its atomic number, the fundamental identifier of each element. The atomic number represents the number of protons in the nucleus, which in turn determines the number of electrons in the atom. Elements with a higher atomic number have more electrons, and thus more valence electrons.
This relationship has profound implications in chemistry. For instance, elements in the same group of the periodic table share the same number of valence electrons, giving them similar chemical properties and bonding tendencies. This observation forms the basis of the periodic law, a guiding principle in understanding the behavior of elements.
Lead and Its Unique Properties
Lead, a fascinating element with atomic number 82, has captured the attention of scientists and engineers for centuries. Its distinctive silvery-white appearance and exceptional malleability and ductility have rendered it vital in a wide array of applications.
Lead’s density, nearly 11 times that of water, grants it remarkable radiation-shielding capabilities. This property has made it indispensable in the construction of medical equipment, nuclear facilities, and protective gear. Its high electrical conductivity further enhances its utility in batteries and electrical cables.
Atomic Number and Valence Electrons
The atomic number of an element, including lead, signifies the number of protons within its nucleus. Protons possess a positive charge, while electrons, found in orbitals surrounding the nucleus, carry a negative charge. The number of valence electrons in an element, those occupying the outermost orbital, profoundly influences its chemical bonding behavior.
Lead’s atomic number of 82 indicates the presence of 82 protons. Consequently, the valence electron count in lead is 4, as the remaining electrons pair within filled inner orbitals. These valence electrons play a pivotal role in lead’s reactivity and bonding capabilities.
Electron Configuration of Lead: Unveiling the Chemical Bonding Potential
Understanding Electron Configuration
Every atom, including the enigmatic lead, possesses a distinct arrangement of electrons around its nucleus. This arrangement, known as electron configuration, dictates the atom’s chemical behavior and bonding capabilities. Electron configuration is represented by a string of numbers and letters that unravel the story of an atom’s electronic structure.
Lead’s Electron Configuration
Lead, with its atomic number 82, harbors a unique electron configuration: [Xe]4f¹⁴5d¹⁰6s²6p². This seemingly complex notation unveils a treasure trove of information. The core, represented by [Xe], denotes lead’s resemblance to the noble gas xenon, with 54 electrons arranged in inner shells. The 4f¹⁴5d¹⁰ notation reveals a fully occupied f-subshell and a d-subshell also brimming with electrons. Finally, the 6s²6p² indicates two electrons in the valence shell—the outermost energy level responsible for chemical bonding.
Valence Electrons: The Key to Chemical Bonding
Valence electrons, those residing in the outermost shell, play a pivotal role in chemical bonding. These electrons determine how an element interacts with others, forming the foundation of chemical reactions and material properties. Lead, with its two valence electrons, is poised to form bonds with other atoms, ensuring its versatility in diverse chemical compounds.
Significance of Valence Electrons in Lead: Unveiling the Secrets of Chemical Bonding
In the realm of chemistry, valence electrons play a pivotal role in determining the behavior and characteristics of elements. Lead, a versatile metal with a wide range of applications, is no exception. Its valence electrons hold the key to understanding its ability to form chemical bonds and contribute to its unique properties.
Valence electrons are the electrons that occupy the outermost energy level of an atom. They are the most loosely bound electrons and, therefore, are the most reactive. In the case of lead, it has four valence electrons. These electrons are responsible for the chemical interactions that lead undergoes, allowing it to form bonds with other atoms and molecules.
The valency of an element refers to the number of chemical bonds that it can form. Lead has a valency of two, indicating that it can form two covalent bonds or two ionic bonds with other elements. This valency is directly related to the number of valence electrons that lead possesses. Each valence electron can participate in the formation of one chemical bond.
The valence electrons of lead play a crucial role in its ability to form various chemical compounds. Lead can form ionic bonds by transferring its valence electrons to other atoms, resulting in the formation of positively charged ions. It can also form covalent bonds by sharing its valence electrons with other atoms, creating strong and stable chemical bonds.
Understanding the significance of valence electrons in lead is essential for comprehending its chemical properties and behavior. The number and arrangement of valence electrons determine the element’s reactivity, bonding capabilities, and ultimately, its suitability for various applications.
Understanding Valence Electrons in Lead
Unveiling the mysteries of chemical bonding requires an in-depth understanding of valence electrons, those pivotal participants in the dance of chemistry. This article embarks on a journey to determine the number of valence electrons in lead, an element with unique properties and applications.
Defining Valence Electrons:
Valence electrons reside in the outermost energy level of an atom, eager to embrace chemical bonding. Their number plays a crucial role in determining an element’s reactivity and chemical behavior. The atomic number of an element, revealing the number of protons in its nucleus, also provides insights into its valence electron count.
Lead: Properties and Applications:
Lead, a heavy and malleable metal, has found diverse applications throughout history, from construction to batteries. Its atomic number, 82, indicates the presence of 82 protons in its nucleus.
Electron Configuration of Lead:
The electron configuration of an element depicts the distribution of its electrons across different energy levels. Lead’s electron configuration, 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 5d¹⁰ 6s² 6p², reveals two valence electrons in its outermost energy level.
Significance of Valence Electrons in Lead:
These two valence electrons empower lead to form chemical bonds with other elements. Lead exhibits a valency of two, implying its ability to share two valence electrons in bonding.
Related Concepts:
-
Atomic Number: The number of protons in an atom’s nucleus, providing a roadmap to its valence electron count.
-
Electron Configuration: A blueprint of an atom’s electron distribution, revealing the number of valence electrons.
-
Chemical Bonding: The dance of valence electrons, forming bonds between atoms to create molecules.
-
Periodic Table: A tapestry of elements, where lead resides in Group 14, indicating its two valence electrons.