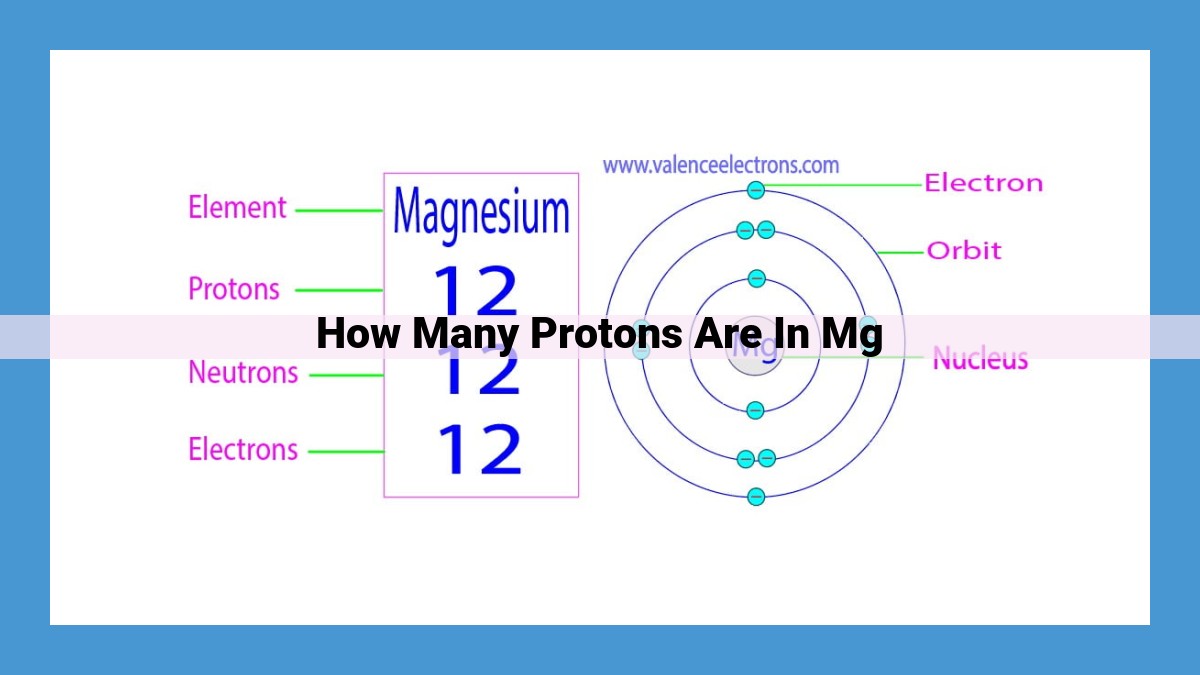
Magnesium (Mg) contains 12 protons in each of its atoms. This is determined by understanding Avogadro’s Number, which relates moles to the number of particles in a substance. Mg’s molar mass helps calculate the mass of a specific amount. The atomic number of Mg, which is 12, indicates the number of protons in each atom. Using these concepts, we can determine that 1 mole of Mg contains 12 moles of protons, which equates to 12 x 6.022 x 10^23 protons.
Unveiling the Number of Protons in Magnesium: A Journey to the Heart of an Atom
Understanding the number of protons in an element is fundamental to comprehending its atomic structure and chemical behavior. In this blog, we embark on a captivating journey to answer the question, “How Many Protons Are in Magnesium?”
We’ll delve into the fascinating world of atoms, exploring key concepts like Avogadro’s Number and molar mass. These concepts lay the groundwork for our quest to determine the number of protons in magnesium, a vital mineral in our bodies.
Setting the Stage: The Importance of Protons
Protons, along with neutrons and electrons, are the building blocks of atoms. The number of protons in an atom defines its atomic number, which determines the element’s identity on the periodic table. Understanding the number of protons in magnesium is crucial for various scientific disciplines, from chemistry to biology.
Avogadro’s Number: The Key to Counting Atoms
In the realm of chemistry, a pivotal concept is Avogadro’s Number. It represents the colossal number of particles (atoms, ions, or molecules) in one mole of a substance, a staggering 6.022 x 10^23. This number serves as a bridge between the microscopic and macroscopic worlds, allowing us to relate the mass and number of particles in a given sample.
Molar Mass: Translating Mass into Number of Particles
Molar mass, measured in grams per mole (g/mol), is another essential concept. It represents the mass of one mole of an element or compound. Knowing the molar mass of magnesium (24.31 g/mol) empowers us to determine the mass of a specific number of magnesium particles.
Atomic Number: Unlocking the Proton Count
The atomic number of an element is a definitive identifier, indicating the number of protons within its atomic nucleus. Magnesium, with an atomic number of 12, reveals that each magnesium atom harbors 12 protons.
Bridging the Gap: Calculating Proton Number
Combining Avogadro’s Number, molar mass, and atomic number, we can unravel the number of protons in magnesium. For a given quantity of magnesium, converting its mass to moles using the molar mass and then multiplying by Avogadro’s Number yields the total number of particles. By further multiplying this value by the atomic number, we arrive at the number of protons in that magnesium sample.
Understanding the number of protons in magnesium sheds light on its atomic structure and explains its unique chemical properties. Whether in the human body, industrial processes, or scientific research, understanding the proton count is essential for unraveling the mysteries of chemistry and the very nature of matter itself.
So, to answer the titular question, “How Many Protons Are in Mg?”, we can confidently assert that each magnesium atom contains 12 protons. This fundamental knowledge empowers us to explore the fascinating world of chemistry with a deeper comprehension of the building blocks of the universe.
Understanding Avogadro’s Number: The Key to Counting Protons in Magnesium
In the world of chemistry, understanding the number of protons in an element is crucial for determining its identity and properties. When it comes to magnesium (Mg), we’re often faced with the question: “How Many Protons Are in Mg?” To answer this, we need to delve into the fascinating concept of Avogadro’s Number.
Avogadro’s Number: The Gateway to Particle Counting
In the realm of chemistry, Avogadro’s Number holds a special place. It represents a mind-boggling number: 6.022 x 10^23. This number is a constant that tells us the exact number of particles present in one mole of a substance. It’s like having a universal counting scale that allows us to measure the tiniest of particles, even those as small as atoms.
The Relationship between Moles, Avogadro’s Number, and Particles
The concept of moles is closely intertwined with Avogadro’s Number. A mole is a unit used to measure the quantity of a substance, just like we use kilograms to measure weight or liters to measure volume. One mole of any substance contains exactly Avogadro’s Number of particles. This means that if we know the number of moles of a substance, we can use Avogadro’s Number to determine the exact number of particles present.
For example, if we have 2 moles of magnesium (Mg), we know that we have 2 x 6.022 x 10^23 = 12.044 x 10^23 magnesium atoms. Avogadro’s Number serves as the bridge that connects the macroscopic world of quantities (moles) to the microscopic world of particles (atoms).
Determining Molar Mass of Magnesium:
- Define molar mass and provide the formula for calculating it
- Explain how molar mass helps determine the mass of a given amount of substance
Determining the Molar Mass of Magnesium
To delve into the realm of determining the number of protons in magnesium (Mg), a crucial step involves understanding its molar mass. Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a substance. It serves as a fundamental conversion factor that enables us to ascertain the mass of a specific quantity of a substance.
The formula for calculating molar mass is:
Molar mass = Sum of atomic masses of all atoms in the formula unit
For magnesium, its atomic mass is approximately 24.31 g/mol. Since magnesium exists as a single atom in its elemental form, its molar mass is simply 24.31 g/mol.
Significance of Molar Mass
Molar mass plays a vital role in determining the mass of a given amount of substance. By multiplying the molar mass by the number of moles present, we can compute the total mass of the substance. This concept is crucial for various chemical calculations, such as determining the mass of reactants or products in chemical reactions.
For instance, if we want to determine the mass of 2 moles of magnesium, we multiply its molar mass (24.31 g/mol) by the number of moles (2), resulting in a mass of 48.62 grams.
Atomic Number of Magnesium: A Gateway to Understanding Protons
In our journey to uncover the mysteries of magnesium’s atomic structure, we encounter a fundamental concept known as the atomic number. This enigmatic value, often denoted by the symbol Z, plays a pivotal role in identifying and understanding elements like magnesium.
The atomic number represents the number of protons residing within each atom of an element. For magnesium, this number stands at 12, indicating that every magnesium atom possesses 12 positively charged protons in its nucleus. Protons, along with their counterparts, neutrons and electrons, constitute the very essence of an atom.
Understanding the atomic number of magnesium provides us with a direct window into the element’s innermost workings. By knowing the number of protons, scientists and researchers can gain insights into the element’s chemical properties, reactivity, and overall behavior.
Calculating the Number of Protons in Magnesium
In our quest to delve into the atomic realm of magnesium (Mg), we must determine the number of protons that reside within each atom. This knowledge unlocks a deeper understanding of the element’s properties and behavior.
To embark on this numerical expedition, we’ll harnesses the power of three fundamental concepts: Avogadro’s Number, Molar Mass, and Atomic Number.
Avogadro’s Number is the astronomical quantity of particles found in one mole of a substance, a concept that provides a bridge between the macroscopic and microscopic worlds. For every mole of Mg, this number is a staggering 6.022 x 10^23.
The Molar Mass of an element represents the mass of one mole of its atoms. In the case of Mg, this value is 24.31 grams per mole. This means that a single mole of Mg atoms collectively weighs 24.31 grams.
Finally, the Atomic Number of an element denotes the number of protons within each atom. For Mg, this number is 12. This fundamental property defines the element’s identity, distinguishing it from all others.
With these concepts firmly in place, we can embark on our calculation. Let’s determine how many protons are present in 1 gram of Mg.
-
Convert grams to moles:
- 1 gram Mg / 24.31 g/mol = 0.0411 moles Mg
-
Multiply moles by Avogadro’s Number:
- 0.0411 moles Mg x 6.022 x 10^23 molecules/mol = 2.47 x 10^22 Mg atoms
-
Multiply Magnesium atoms by Atomic Number:
- 2.47 x 10^22 Mg atoms x 12 protons/atom = 2.96 x 10^23 protons
So, with this meticulously crafted calculation, we have established that there are 2.96 x 10^23 protons in 1 gram of magnesium.