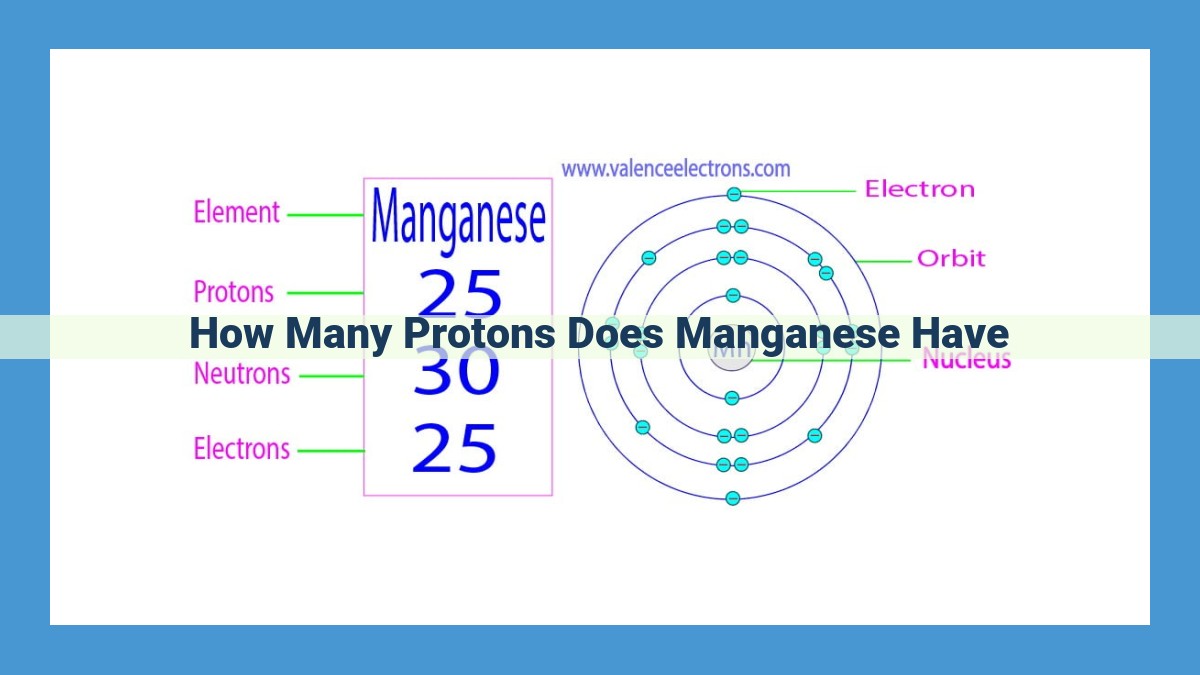
Manganese, a versatile transition metal, has 25 atomic number. Atomic number refers to the number of protons in the atom’s nucleus and defines an element’s identity. Manganese’s atomic number places it in the periodic table, where the organization based on atomic number allows for the prediction of its properties. Transition metals like manganese exhibit specific traits such as hardness, ductility, and high conductivity.
**Atomic Structure: Unveiling the Building Blocks of Matter**
Imagine stepping into a bustling metropolis, where every individual plays a distinct role in the city’s symphony. Just as this urban tapestry is made up of countless citizens, so too is every speck of matter composed of fundamental particles called atoms. These atomic building blocks are the very essence of our universe, shaping our physical world.
At the heart of every atom lies its nucleus, a dense, positively charged core. Here reside the protons, tiny particles that carry a positive charge. Cuddling up with the protons are neutrons, their neutral cousins that add weight to the nucleus.
Surrounding the nucleus like a celestial entourage are electrons, lightweight particles that carry a negative charge. These electrons dance around the nucleus in designated paths, creating an electron cloud. This cloud determines the atom’s chemical properties and its interactions with other atoms.
Together, protons, neutrons, and electrons form the smallest unit of an element, the atom. Each element, from hydrogen to gold, has a unique atomic number, which is simply the number of protons in its nucleus. This atomic number defines the element’s identity and its place in the periodic table.
Manganese: A Versatile Transition Metal
In the vast tapestry of elements that shape our world, there exists a remarkable metal called manganese. Its story is intertwined with the fundamental building blocks of matter itself, making it an integral part of our scientific and technological advancements.
Manganese’s Atomic Fingerprint
At the heart of every atom lies a nucleus, containing protons and neutrons, while electrons dance around it. The number of protons defines an element’s identity, like a unique fingerprint. Manganese proudly bears the atomic number 25, meaning it possesses 25 protons within its nucleus. This unique atomic signature governs the element’s chemical behavior and its place within the periodic table.
A Periodic Haven
The periodic table serves as a vibrant map of the elements, arranging them in an orderly manner based on their atomic numbers. Manganese resides in Group 7, Period 4, among the transition metals, a group renowned for their exceptional properties.
Transition Metal Traits
Transition metals are characterized by their hardness and ductility. They possess high melting points and exhibit excellent conductivity, making them invaluable in countless applications. Manganese, true to its transition metal nature, embodies these traits, contributing to its versatility and usefulness in various industries.
Manganese’s Natural Abode
Nature, in its infinite wisdom, has generously bestowed upon us an abundance of manganese. It is found in various minerals, including pyrolusite (MnO2), rhodochrosite (MnCO3), and manganite (MnOOH). These minerals serve as the primary sources for extracting this versatile metal, which has made its mark in a wide array of applications.
Atomic Number: The Identity Card of an Element
In the vast tapestry of the universe, matter takes countless forms, each with its unique identity. At the heart of this diversity lies the atom, the fundamental building block of all matter. One crucial aspect that defines an atom’s distinctiveness is its atomic number, a number that holds the key to understanding its elemental nature.
The atomic number represents the number of positively charged particles, called protons, residing within an atom’s nucleus. These protons, along with the electrically neutral neutrons, determine the mass of the atom. It’s like an identity card that each element carries, revealing its distinctive features.
The atomic number plays a pivotal role in shaping an element’s chemical properties. It dictates the number of electrons that orbit the nucleus, which in turn determines the element’s reactivity and behavior in chemical reactions. For instance, elements with a high atomic number tend to be more reactive, easily forming chemical bonds with other elements.
The periodic table, an ingenious arrangement of elements, is organized primarily based on atomic number. Elements with similar atomic numbers are grouped together, revealing patterns and trends in their properties. This arrangement allows scientists to predict the properties of unknown elements based on their position within the table.
For example, manganese (atomic number 25) is classified as a transition metal, a group of elements known for their exceptional hardness, ductility, and high melting points. Its atomic number places it alongside other transition metals like iron, nickel, and copper, suggesting that it shares similar characteristics, such as electrical conductivity and the ability to form alloys.
Understanding atomic number is fundamental to comprehending the nature of elements and their behavior in chemical reactions. It’s like a compass that guides us through the vast elemental landscape, unlocking the secrets of each element’s identity and properties.
The Periodic Table: A Road Map to the Elements
In the vast labyrinth of chemistry, the Periodic Table stands as an illuminating beacon, guiding us through the maze of elements that make up our world. This ingenious chart, organized by atomic number, unveils the fundamental relationships and properties of each element.
With atomic number as our guiding compass, the periodic table arranges elements horizontally into periods, and vertically into groups. Elements belonging to the same group share a distinctive set of characteristics, such as the number of valence electrons, which influence their chemical behavior.
By venturing through the periodic table, we uncover a treasure trove of information. The elements’ atomic radii increase as we move down a group, while their ionization energies diminish. Electronegativity, the tendency to attract electrons, generally increases as we move across a period.
But the periodic table doesn’t merely catalog elements; it grants us the power to predict their properties. By observing the location of an element, we can infer its chemical reactivity, physical characteristics, and even its potential uses. This predictive power makes the periodic table an indispensable tool for scientists, engineers, and anyone seeking to unravel the intricacies of the chemical world.
Manganese’s Place in the Periodic Table
- Specify manganese’s atomic number (25) and its position in the periodic table.
Manganese’s Place in the Periodic Table
In the vast tapestry of elements, manganese stands out as a versatile transition metal with unique properties. As we venture into the heart of the periodic table, let’s unravel the secrets of manganese’s identity and its pivotal role within this extraordinary framework of elements.
Manganese occupies the 25th slot in the periodic table, where each element is meticulously arranged based on its atomic number—the number of protons in its nucleus. This atomic number is what defines an element’s identity and, consequently, its chemical properties.
The periodic table is not merely a static display but a dynamic guide that allows us to predict element properties based on their location. By observing the patterns and trends within the table, scientists can deduce key characteristics of elements even before encountering them in nature.
Manganese’s place in the periodic table reveals its status as a transition metal, a group of elements renowned for their exceptional hardness, ductility, and high melting points. These properties make transition metals indispensable for a wide array of applications, from construction to electronics.
In conclusion, manganese’s precise positioning in the periodic table grants us invaluable insights into its atomic structure, properties, and chemical behavior. This knowledge enables us to harness manganese’s unique characteristics in countless ways, contributing to technological advancements and improving our understanding of the fundamental building blocks of the universe.
Transition Metals: A Unique Group
Transition metals, a diverse family in the periodic table, hold a special place in the world of elements. Their atomic structures, characterized by partially filled d orbitals, endow them with a range of distinctive properties.
Hardness and Ductility
Transition metals are renowned for their hardness, giving them the ability to withstand scratches and deformations. Their ductility, on the other hand, allows them to be stretched and shaped without breaking. This combination of strength and flexibility makes them ideal for applications in construction, machinery, and transportation.
High Melting Points
Transition metals possess high melting points, indicating the strong atomic bonds holding them together. This property makes them suitable for high-temperature applications, such as in furnaces, boilers, and turbines. Their stability under intense heat ensures reliable performance and longevity in demanding environments.
Good Conductivity
Transition metals are excellent conductors of heat and electricity. Their partially filled d orbitals facilitate the flow of electrons, allowing them to efficiently transfer energy and information. This property has led to their widespread use in electrical systems, electronic devices, and thermal applications.
In summary, transition metals owe their unique properties to their atomic structures and partially filled d orbitals. Their hardness, ductility, high melting points, and good conductivity make them indispensable in a myriad of industrial, technological, and everyday applications.