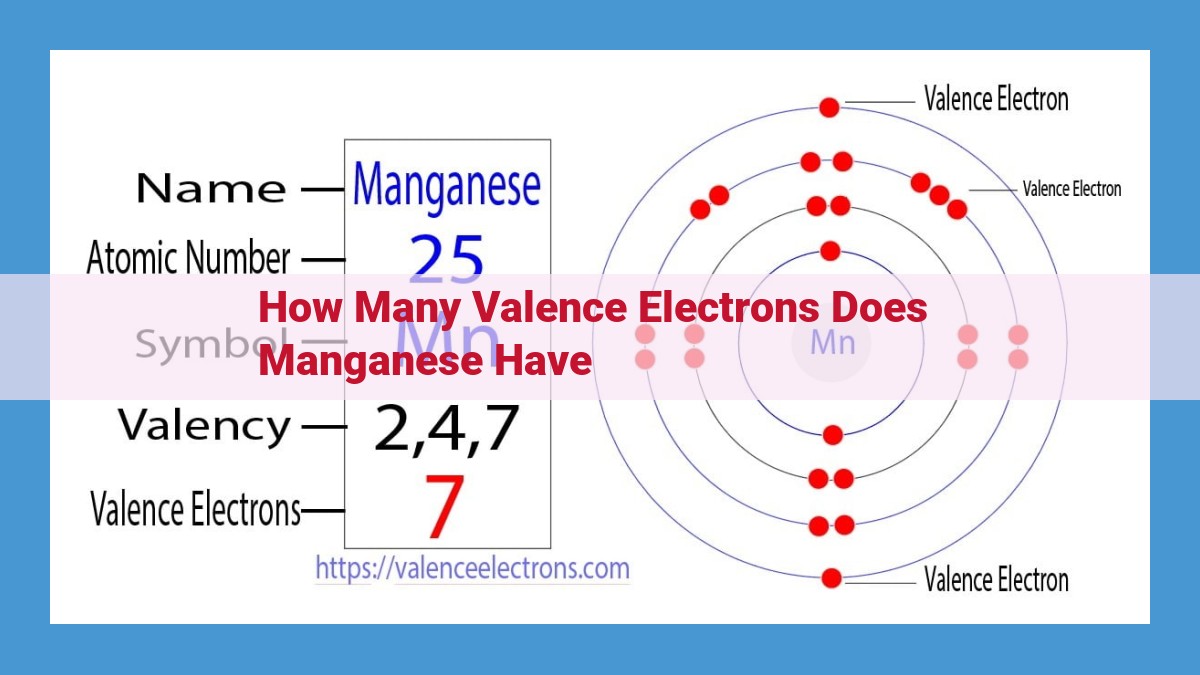
Manganese, renowned for its significance in chemistry, boasts 25 electrons, with 7 of them being valence electrons. Residing in the outermost energy level, these valence electrons play a crucial role in determining manganese’s chemical behavior. Their presence influences the element’s oxidation states, reduction potential, and ability to form chemical bonds. Understanding the concept of valence electrons is paramount in comprehending manganese’s involvement in various chemical reactions and its overall reactivity.
Unlocking the Secrets of Manganese’s Valence Electrons: A Chemical Odyssey
Have you ever wondered about the inner workings of manganese, that fascinating element that plays a crucial role in our world? One key aspect of understanding manganese’s behavior lies in its valence electrons, the electrons that determine its chemical interactions. Join us on a captivating journey to delve into the mysteries of manganese’s valence electrons and their profound significance.
Manganese: The Element with a Storied Past
Manganese, with its atomic number of 25, has captivated scientists and chemists for centuries. Its name, derived from the Greek word “magnes”, translates to “magnet stone”, hinting at its magnetic properties. But beyond its magnetic allure, manganese holds a trove of other secrets, including the enigmatic nature of its valence electrons.
Electron Configuration: The Blueprint for Chemical Behavior
Every atom possesses a unique arrangement of electrons, known as its electron configuration. For manganese, this configuration unfurls as:
[Ar] 3d5 4s2
This intricate arrangement reveals that manganese’s valence electrons reside in the outermost energy level, the 4s orbital. These seven valence electrons are the key to understanding manganese’s chemical behavior.
Valence Electrons: The Gatekeepers of Chemical Reactions
Valence electrons play a pivotal role in chemical bonding, the process by which atoms combine to form molecules. They determine an element’s ability to share or exchange electrons, forming the bonds that hold matter together. Manganese’s abundance of valence electrons makes it a versatile player in chemical reactions, enabling it to participate in a wide range of interactions.
Applications and Significance: Uniting Science and Industry
The understanding of manganese’s valence electrons extends far beyond the confines of academic curiosity. It has profound implications in various fields:
-
Oxidation states and reduction potential: Valence electrons determine an element’s ability to lose or gain electrons, influencing its oxidation state and reduction potential. This knowledge is crucial in electrochemical reactions and battery technologies.
-
Chemical bonding and reactivity: Valence electrons govern the types of bonds an element can form and its overall chemical reactivity. This understanding guides the design and synthesis of new materials and compounds.
-
Understanding manganese chemistry: Valence electrons provide insights into the behavior and properties of manganese-containing compounds, which find applications in diverse areas such as metallurgy, catalysis, and medicine.
Manganese Atomic Number: A Gateway to Its Chemical Behavior
In the realm of chemistry, the atomic number of an element is paramount, as it dictates its fundamental characteristics. Manganese, with an atomic number of 25, holds a special place in the periodic table, unveiling the secrets behind its intriguing chemical behavior.
The atomic number, often denoted by the symbol Z, represents the number of protons residing in the nucleus of an atom. Protons carry a positive charge, while electrons, found in the surrounding orbitals, bear negative charges. In a neutral atom, the number of protons equals the number of electrons, thus balancing the electrical forces.
Manganese’s atomic number of 25 signifies that its nucleus contains 25 protons, making it a positively charged entity. This fundamental property underscores the element’s distinctive chemical behavior. It determines the number of electrons that orbit the nucleus, which in turn influences manganese’s ability to form chemical bonds.
Manganese’s Electron Configuration: Unveiling the Secret to Its Chemical Behavior
Imagine you’re a detective, eager to unravel the mysteries surrounding manganese, a fascinating element with a multitude of intriguing characteristics. One of the keys to understanding manganese’s behavior lies in its electron configuration, a roadmap that reveals the arrangement of its electrons and governs its chemical interactions.
Let’s delve into the concept of electron configuration. Each atom, including manganese, consists of a nucleus surrounded by electrons. Electrons occupy specific energy levels, arranged in shells and subshells. The electron configuration tells us the number of electrons in each subshell.
Manganese’s electron configuration, designated as [Ar] 3d5 4s2, resembles a secret code. The [Ar] part indicates that manganese has the same electron configuration as the noble gas argon, with 18 electrons in its innermost shells. This stable configuration creates a foundation upon which manganese’s unique characteristics are built.
The 3d5 subshell holds five electrons, making it partially filled. This partial filling gives manganese its transition metal status, granting it the ability to exhibit multiple oxidation states. The 4s2 subshell contains two valence electrons, the electrons involved in chemical bonding. These valence electrons determine manganese’s reactivity and shape its interactions with other elements.
Understanding manganese’s electron configuration is essential for unraveling its chemical mysteries. It unveils the element’s potential for forming various bonds, its ability to change oxidation states, and its overall behavior in chemical reactions. As we continue our detective work, deciphering manganese’s electron configuration will lead us closer to solving the enigma of this intriguing element.
Valence Electrons: The Secret to Chemical Bonding
In the realm of chemistry, the dance between atoms is orchestrated by the valence electrons, the enigmatic characters that determine how elements interact with each other. Manganese, a silvery-white metal, plays a fascinating role in this atomic ballet, possessing a unique arrangement of valence electrons that grants it remarkable chemical properties.
Imagine the atom as a miniature solar system, with electrons orbiting the nucleus like planets. These electrons are arranged in concentric shells, each with a specific energy level. The valence electrons are those inhabiting the outermost shell, the most energetic and influential in determining an element’s chemical behavior.
In the case of manganese, its atomic number of 25 reveals that it has a total of 25 electrons. Its electron configuration describes the distribution of these electrons within the shells: [Ar] 3d5 4s2. This arrangement indicates that manganese has seven valence electrons residing in the outermost 3d and 4s orbitals.
These seven valence electrons are the key players in manganese’s chemical reactions. They determine the element’s oxidation states, the charges it can assume when bonding with other atoms. Manganese’s versatile valence electrons enable it to form various compounds, showcasing its diverse chemical nature.
The dance of valence electrons not only shapes the reactivity of manganese but also influences its magnetic properties. The unpaired valence electrons in manganese’s 3d orbitals contribute to its magnetism, making it a useful material in magnets and other technological applications.
Understanding the world of valence electrons is crucial for unraveling the secrets of manganese chemistry. It provides a window into the element’s interactions, allowing us to predict its behavior and harness its potential in various scientific and industrial fields.
Manganese: Unraveling the Secrets of Valence Electrons
Prepare to delve into the fascinating world of manganese and uncover the mysteries surrounding its valence electrons. These electrons hold the key to unlocking manganese’s chemical behavior and understanding its significance in various applications.
Manganese’s Identity
Every element is defined by its atomic number, which represents the number of protons within its nucleus. In the case of manganese, this number is 25, indicating the presence of 25 protons. Since atoms maintain electrical neutrality, the number of electrons also equals 25.
Delving into the Electron Configuration
The arrangement of electrons within an atom is known as its electron configuration. For manganese, it takes the form of [Ar] 3d5 4s2. This notation indicates that manganese has:
- 18 electrons in its core, similar to argon (Ar).
- Five electrons in its 3d subshell.
- Two electrons in its outermost 4s subshell.
Valence Electrons: The Chemical Bridge
Valence electrons are the electrons in the outermost subshell, which are the most influential in chemical bonding. Manganese possesses seven valence electrons, giving it a high tendency to participate in chemical reactions. These electrons determine manganese’s ability to form bonds, share electrons, and undergo redox reactions.
Unveiling the Significance
Manganese’s valence electrons have far-reaching implications in the element’s chemistry:
- Oxidation States and Reduction Potential: The number of valence electrons influences manganese’s tendency to lose or gain electrons, affecting its oxidation states and reduction potential.
- Chemical Bonding and Reactivity: Valence electrons facilitate chemical bonding by forming bonds with other atoms. They also influence manganese’s reactivity with various elements.
- Manganese Chemistry Fundamentals: Understanding manganese’s valence electrons is essential for comprehending the element’s overall chemical behavior and its role in biological and industrial applications.
Manganese’s seven valence electrons are not mere spectators but active participants in the element’s chemical playground. They govern manganese’s bonding behavior, redox properties, and overall reactivity. By unraveling the secrets of valence electrons, we unlock the key to understanding manganese chemistry, its applications, and its profound significance in the world around us.