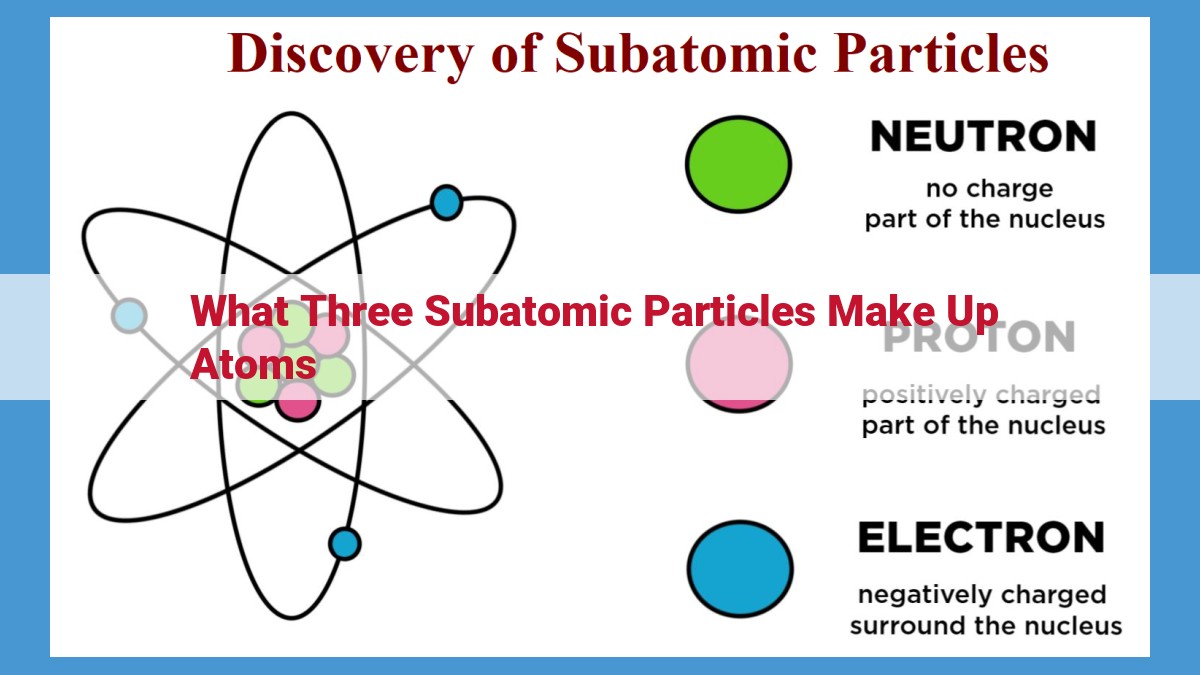
Atoms, the fundamental building blocks of all matter, are composed of three subatomic particles: protons, neutrons, and electrons. Protons, residing in the nucleus, carry a positive charge, determining an atom’s atomic number. Neutrons, also in the nucleus, are neutral, contributing to an atom’s mass. Electrons, orbiting the nucleus, carry a negative charge, neutralizing the overall charge and balancing the number of protons. These three particles, with their distinct charges and locations, constitute the fundamental trio that defines the structure of every atom.
Atoms: The Fundamental Building Blocks of Our Universe
In the realm of matter that constructs our world and beyond, there exist atoms, the building blocks of everything we know. These microscopic marvels are so small that millions could fit comfortably on the tip of a pen. Yet within these tiny structures lies a universe of its own, teeming with activity.
Comprising these atoms are three fundamental subatomic particles: protons, neutrons, and electrons. Each particle plays a unique role in defining the properties of the atom, like a harmonious trio working together to create the diverse matter that surrounds us.
Protons: The Positively Charged Nucleus
Imagine an atom, the fundamental building block of matter. Within its tiny heart lies a nucleus, the core that holds the atom’s secrets. One of its three vital components is the proton, a tiny particle carrying a positive charge.
Protons are found nestled within the nucleus, along with their neutral counterparts, neutrons. Together, they form the nucleus, the dense center of the atom where the majority of its mass resides.
The number of protons within an atom’s nucleus determines its atomic number. Each element, from hydrogen to uranium, has a unique atomic number that identifies it on the periodic table. This number is equal to the number of electrons orbiting the nucleus, ensuring the atom is electrically neutral.
Protons play a crucial role in defining the characteristics of an element. They influence the chemical reactions an element can undergo, its physical properties, and its place in the periodic table.
For example, hydrogen has one proton, helium has two, and oxygen has eight. These numbers determine their chemical reactivity, with hydrogen being the most reactive and oxygen being less reactive.
So, protons are the positively charged electrical giants that reside in the nucleus of an atom, dictating its atomic number and shaping its identity within the vast tapestry of elements that make up our world.
Neutrons: The Silent Guardians of the Nucleus
Every atom, the building block of the universe, is composed of a fundamental trio of subatomic particles: protons, neutrons, and electrons. While protons and electrons receive much attention, neutrons often remain the enigmatic, silent guardians of the atomic nucleus. This article unveils the fascinating world of neutrons, exploring their unique characteristics and their crucial role in shaping the atoms around us.
The Neutral Core:
Nestled alongside the positively charged protons within the heart of the atom, neutrons are aptly named due to their neutral electrical charge. Unlike their charged counterparts, they carry neither positive nor negative charges, rendering them invisible to the electric forces that govern atomic interactions.
Mass Matters:
Neutrons contribute significantly to an atom’s mass. Together with the protons, they determine the mass number of an atom, which represents the total number of nucleons (protons and neutrons) within the nucleus. This mass number plays a crucial role in identifying isotopes, atoms of the same element with varying numbers of neutrons.
Isotopic Variations:
The number of neutrons in an atom can vary, giving rise to different isotopes of the same element. For instance, carbon-12, with six protons and six neutrons, is the common isotope we encounter in nature. However, carbon-14, with six protons and eight neutrons, is a radioactive isotope used in carbon dating.
Neutrons, often overlooked in the shadow of protons and electrons, are essential components of atoms. Their neutral charge stabilizes the nucleus, while their mass contributes significantly to the atom’s overall weight. Isotopic variations, caused by varying neutron counts, add complexity and diversity to the atomic world. Understanding the role of neutrons unveils a deeper appreciation for the intricate structure and properties of matter.
Electrons: The Negatively Charged Orbiters
- Describe electrons as negatively charged particles orbiting the nucleus.
- Explain that their number matches the number of protons, neutralizing the atom’s overall charge.
Electrons: The Negatively Charged Orbiters
In the realm of subatomic wonders, electrons reign as the negatively charged entities that dance around the nucleus of an atom. These tiny particles are crucial in maintaining the atom’s balance and defining its properties.
Electrons in Orbit
Imagine electrons as miniature celestial bodies, orbiting the nucleus like planets around a star. They occupy specific energy levels, creating distinct shells or orbitals within the atom. The closer an electron is to the nucleus, the lower its energy level and the stronger its attraction to the positively charged protons.
Matching the Proton Count
One remarkable aspect of electrons is their ability to neutralize the atom’s overall charge. Atoms contain an equal number of electrons and protons, resulting in a neutral state. This delicate balance ensures that atoms do not possess an electrical charge and can interact with other atoms without interference from excess charges.
Electron Distinctiveness
Electrons play a vital role in determining the chemical behavior of atoms. Their arrangement in shells influences the atom’s valence, which governs its ability to form chemical bonds with other atoms. Moreover, electrons are responsible for the atom’s electrical and thermal conductivity, as well as its magnetic properties.
In the subatomic world, electrons stand as fundamental building blocks of matter. Their negative charge, orbital motion, and matching proton count are essential elements in maintaining the stability and defining the properties of atoms. Understanding the role of electrons provides a foundation for unraveling the intricacies of chemistry and other scientific disciplines.