To determine percent recovery, calculate the theoretical yield using stoichiometry, then measure the actual yield experimentally. Calculate percent recovery using the formula: (Actual Yield / Theoretical Yield) x 100%. Ideal percent recovery is 100%, with deviations indicating recovery loss due to factors such as incomplete reactions, side reactions, or experimental errors. Optimizing production processes can improve percent recovery, ensuring efficient utilization of reactants and accurate product yields.
- Define percent recovery and explain its significance in chemical reactions.
- Discuss the relationship between theoretical yield, actual yield, and percent recovery.
The Importance of Percent Recovery in Chemical Reactions
In the world of chemistry, percent recovery is a crucial parameter that measures the efficiency of a chemical reaction. It provides valuable insights into how much of a desired product we actually obtain compared to the amount we theoretically expect.
Percent recovery is defined as the ratio of actual yield (the amount of product we get in practice) to theoretical yield (the amount we should get based on stoichiometry) multiplied by 100%. Understanding percent recovery is essential because it helps us assess the effectiveness of our reactions and identify areas for improvement.
The relationship between theoretical yield, actual yield, and percent recovery is straightforward:
- Theoretical yield represents the maximum amount of product that can be formed based on the stoichiometry of the reaction. It assumes perfect reaction conditions and no losses.
- Actual yield is the amount of product we actually obtain, which is often less than theoretical yield due to various factors such as incomplete reactions, side reactions, and experimental errors.
- Percent recovery reflects the efficiency of our reaction by expressing the actual yield as a percentage of the theoretical yield.
Calculating Theoretical Yield: The Foundation of Percent Recovery
In the realm of chemical reactions, understanding the theoretical yield is like possessing a blueprint, guiding us towards the maximum amount of product that can be produced under perfect conditions. This elusive ideal quantity is calculated using the principles of stoichiometry, the language of chemical proportions.
Stoichiometry, the meticulous art of balancing chemical equations, provides a roadmap to determine the exact amounts of reactants and products involved in a reaction. It’s like a chemical recipe book, telling us how many atoms or molecules of each substance are required and produced.
To calculate the theoretical yield, we embark on a two-step journey. First, we analyze the balanced chemical equation, which reveals the exact stoichiometric ratios between reactants and products. This equation serves as a roadmap for the reaction, dictating the proportions of each substance.
Armed with this knowledge, we embark on the second step: applying these ratios to the initial amount of reactants. Let’s say we want to calculate the theoretical yield of water produced when hydrogen and oxygen react. The balanced equation is:
2H₂ + O₂ → 2H₂O
This equation tells us that 2 molecules of hydrogen react with 1 molecule of oxygen to produce 2 molecules of water. If we start with, say, 1 mole of hydrogen, we can use the ratio to determine how much oxygen is needed and the corresponding amount of water produced.
Simple stoichiometric calculations like these empower us to predict the theoretical yield, the maximum amount of product that can be obtained under ideal conditions. This value serves as a benchmark against which we can compare the actual yield, a testament to the efficiency of our chemical endeavors and a valuable tool for optimizing production processes.
Determining Actual Yield
Measuring Actual Yield
Determining the actual yield involves measuring the quantifiable amount of product obtained from a chemical reaction. This measurement is typically conducted experimentally, using techniques such as gravimetric analysis (weighing) or volumetric methods (measuring volume).
Factors Affecting Actual Yield
Several experimental factors can influence the actual yield of a reaction. These include:
- Purity of reactants: Impurities can interfere with the reaction, reducing product formation.
- Reaction conditions: Temperature, pressure, and reaction time can all affect the rate and extent of the reaction.
- Efficiency of the separation methods: Incomplete separation of the product from impurities or side products can result in a lower actual yield.
Common Sources of Error
Errors in measuring actual yield can arise from various sources:
- Measurement inaccuracies: Imprecise equipment or human error can lead to erroneous measurements.
- Losses during transfer: Product loss can occur during transfer between containers or instruments.
- Incomplete reactions: The reaction may not have proceeded to completion, resulting in a lower actual yield.
Minimizing Errors
To minimize the impact of errors on actual yield, it is crucial to:
- Calibrate equipment regularly: Ensuring that measuring devices are accurate and functional.
- Handle materials carefully: Avoid spills or losses during transfer and manipulation.
- Optimize reaction conditions: Determine the ideal conditions for the reaction to maximize product formation.
- Employ efficient separation methods: Ensure complete separation of the product from impurities or side products.
Percent Recovery: A Crucial Aspect of Chemical Reactions
Understanding Percent Recovery
In chemical reactions, percent recovery is a key measure that quantifies the efficiency of a process. It represents the proportion of the theoretical yield that is actually obtained in the experiment.
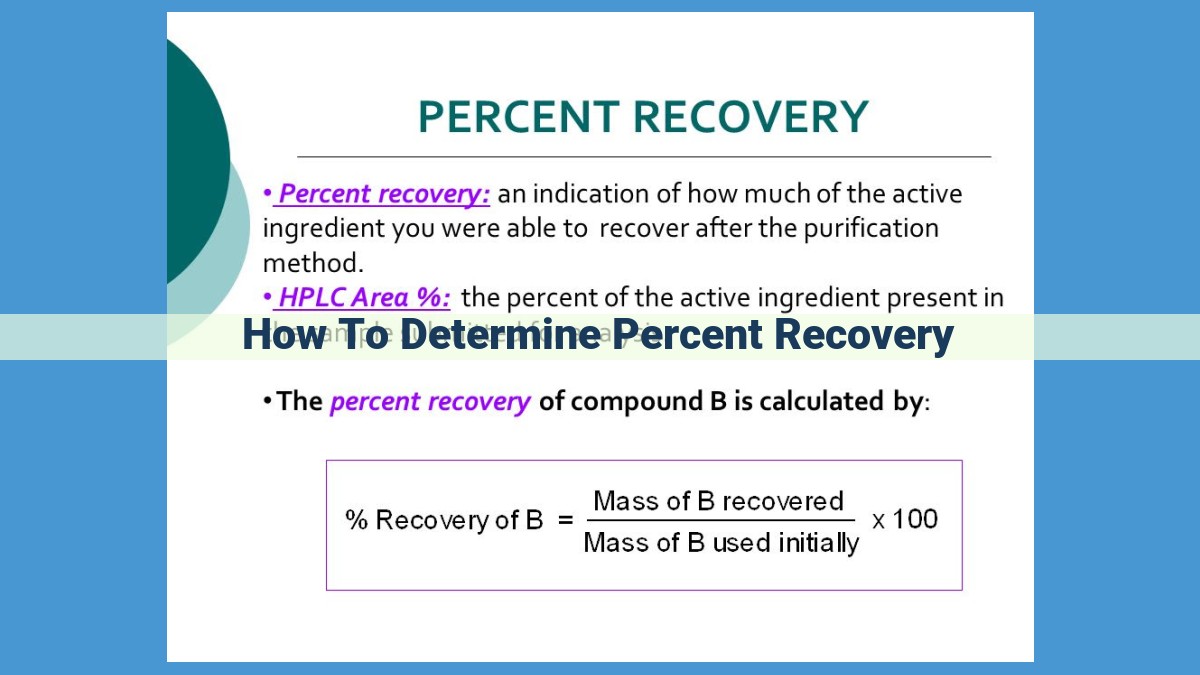
Calculating Percent Recovery: The Formula
Percent recovery is calculated using the following formula:
Percent Recovery = (Actual Yield ÷ Theoretical Yield) × 100%
Actual Yield
Actual yield refers to the amount of product that is physically recovered from the reaction after completion. It is determined by experimental measurements, such as mass, volume, or spectroscopic techniques.
Theoretical Yield
Theoretical yield is the maximum amount of product that could be obtained if the reaction proceeded with 100% efficiency. It is calculated using stoichiometry, which involves analyzing the balanced chemical equation to determine the ideal molar ratios of reactants and products.
The Importance of Percent Recovery
Percent recovery provides valuable insights into the success and efficiency of a chemical process.
- High Percent Recovery (close to 100%): Indicates that the reaction proceeded efficiently, with minimal losses due to side reactions, incomplete reactions, or errors.
- Low Percent Recovery (significantly below 100%): Signals potential problems with the reaction process, such as incomplete reactions, side reactions, or experimental errors. It prompts further investigation to optimize production.
Interpreting Percent Recovery
Determining the percent recovery of a chemical reaction grants invaluable insights into the efficiency and efficacy of your processes. Aiming for an optimal percent recovery is paramount, as it indicates minimal losses and efficient utilization of reactants. However, deviations from this ideal value can provide crucial clues to underlying issues.
Common Deviations from Optimal Percent Recovery
-
Percent recovery < 100%: This indicates incomplete reactions, losses during isolation or purification, or incorrect stoichiometry.
-
Percent recovery > 100%: Although seemingly desirable, this is unlikely and may suggest experimental errors, contamination, or impurities in the product.
Identifying Sources of Recovery Loss
By analyzing the percent recovery data and considering the specific reaction parameters, it’s often possible to pinpoint the source(s) of recovery loss:
-
Low starting concentrations: Starting with lower reactant concentrations can result in decreased actual yield and, subsequently, lower percent recovery.
-
Ineffective reaction conditions: Factors such as temperature, pressure, pH, or catalyst presence can significantly influence reaction efficiency.
-
Incomplete reactions: Ensuring complete conversion of reactants can be crucial for maximizing percent recovery.
-
Losses during isolation or purification: Careful handling and appropriate techniques during these steps are essential to minimize product loss.
Optimizing Production Processes
Identifying the underlying causes of recovery loss empowers you to develop strategies for optimizing your production processes and enhancing percent recovery:
-
Adjusting reaction conditions: Fine-tuning temperature, pressure, pH, and other parameters can improve reaction efficiency.
-
Employing more effective catalysts: Catalysts can accelerate reactions and promote complete conversion.
-
Refining isolation and purification techniques: Optimizing these steps minimizes product loss and improves yield.
-
Eliminating impurities: Identifying and removing impurities from reactants or products can enhance purity and percent recovery.