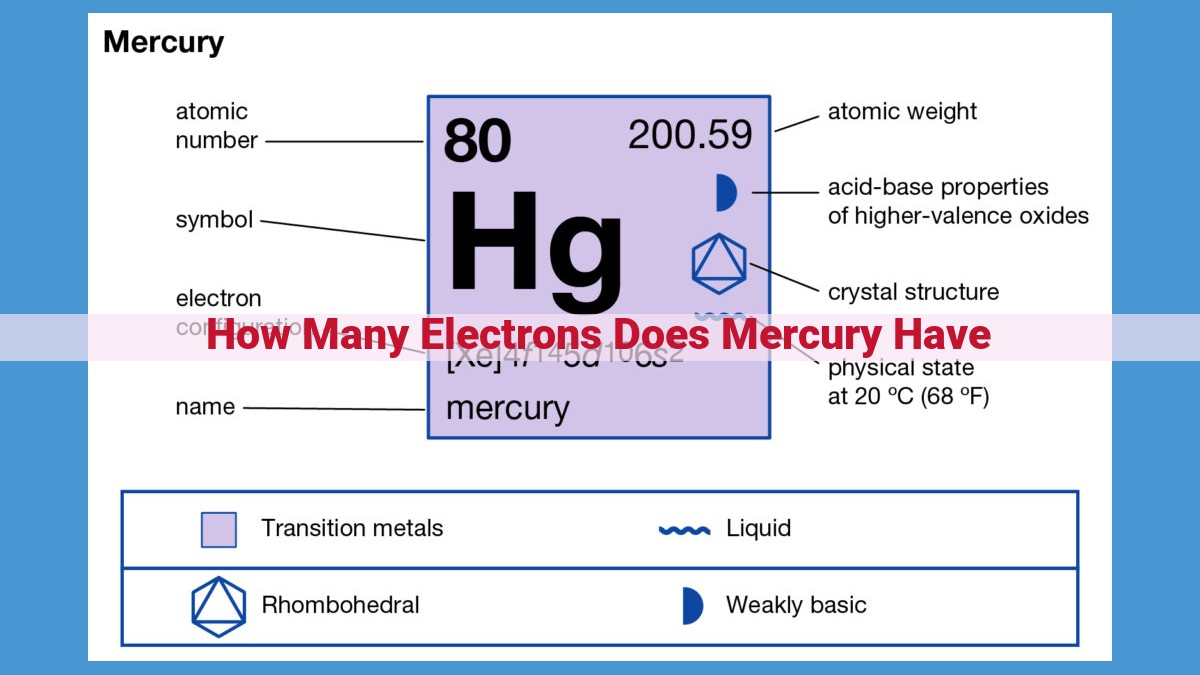
Mercury, a heavy metal with atomic number 80, possesses a unique electron configuration. It has a total of 80 electrons distributed across energy levels or shells. The electrons in the outermost shell, known as valence electrons, play a crucial role in determining its chemical properties. Mercury has 2 valence electrons, which occupy the 6th energy level. This electronic structure places mercury in Group 12 of the periodic table, influencing its bonding behavior and reactivity.
Unveiling the Electronic Secrets of Mercury: A Guide to Electron Configuration
In the realm of chemistry, the electron configuration of an element holds immense importance in understanding its chemical properties. For mercury, this configuration plays a crucial role in determining its valence electrons, which are the outermost electrons responsible for chemical bonding and reactivity.
Energy Levels and Shells: The Orbital Architecture of Mercury
Imagine electrons as tiny particles residing in specific energy levels around the atom’s nucleus. These energy levels form concentric circles called shells, with each shell holding a maximum number of electrons. The first shell can accommodate up to 2 electrons, the second shell up to 8 electrons, and so on.
Electron Configuration of Mercury: A Story of 80 Electrons
Mercury possesses 80 electrons, which are distributed in different shells and energy levels. The first shell is filled with 2 electrons, followed by 8 electrons in the second shell. The third shell contains 18 electrons, the fourth shell has 32 electrons, and the fifth shell has 18 electrons. The remaining 2 electrons occupy the sixth shell, in an energy level known as the 6s orbital.
Valence Electrons: Mercury’s Chemical Fingerprint
The valence electrons are those found in the outermost shell, which is the 6s orbital for mercury. These 2 valence electrons are crucial for chemical bonding and determine the element’s reactivity. The presence of only 2 valence electrons places mercury in Group 12 of the periodic table, known as the “d-block” elements.
Atomic Number of Mercury
Every atom possesses a unique identity defined by its atomic number. This number represents the quantity of positively charged protons residing within the atomic nucleus. The atomic number plays a pivotal role in determining an element’s position on the periodic table and its chemical properties.
In the case of mercury, its atomic number is 80. This signifies that each mercury atom contains 80 protons within its nucleus. These protons are balanced by an equal number of negatively charged electrons, resulting in a neutral overall charge for the atom.
The atomic number is a fundamental property that distinguishes one element from another. It governs the number of electrons an atom possesses, which in turn determines its chemical reactivity. Elements with similar atomic numbers tend to exhibit comparable chemical behaviors, forming the basis for the periodic table’s organization.
For mercury, its position in Group 12 of the periodic table is attributed to its atomic number of 80. Elements within the same group share similar valence electron configurations, which are the electrons occupying the outermost energy level. In mercury’s case, it has two valence electrons, reflecting its placement in Group 12.
Determining the Number of Valence Electrons in Mercury
delve into the fascinating world of chemistry, where the properties of elements are governed by their electron configurations. Today’s spotlight shines on mercury, an enigmatic element with a lustrous silvery appearance and a rich history in science and industry.
Understanding Valence Electrons
In the realm of chemistry, valence electrons play a pivotal role. These are the electrons that inhabit the outermost energy level of an atom, eagerly participating in chemical reactions and determining the element’s chemical properties. Picture these valence electrons as the social butterflies of the atomic world, always seeking interaction with others.
Calculating Mercury’s Valence Electrons
To determine the number of valence electrons in mercury, we embark on a mathematical escapade. Mercury’s atomic number, which uniquely identifies it on the periodic table, is 80. This means that mercury has a total of 80 electrons buzzing around its nucleus.
Now, we need to account for the electrons residing in the inner shells. These electrons are content to remain close to the nucleus and don’t participate directly in chemical interactions. Mercury has 78 inner-shell electrons, leaving us with 80 – 78 = 2 valence electrons.
Mercury’s Position in the Periodic Table
Mercury finds its home in Group 12 of the periodic table, also known as the “d-block.” This placement has a profound impact on the element’s valence electrons. d-block elements typically have valence electrons in their d orbitals, which are located just beneath the outermost s orbital.
Our exploration of mercury’s electron configuration has led us to the discovery that it possesses two valence electrons. This understanding is crucial for comprehending the chemical behavior of mercury and its ability to form compounds with other elements. The electron configuration and atomic number of an element are essential pieces of information that provide valuable insights into its properties.
The Influence of Atomic Number on Valence Electrons
As we embark on this scientific adventure, let’s unveil the secrets of mercury’s electron configuration and its significance in determining the number of valence electrons.
Atomic Number: The Fingerprint of an Element
Every element in our vast universe is unique, possessing a distinctive atomic number. This number represents the number of protons residing within the element’s nucleus. For our protagonist, mercury, this number is 80. This atomic number plays a pivotal role in understanding mercury’s behavior.
Periodic Trend: A Journey Across the Table
The Periodic Table serves as a roadmap for all known elements, arranging them in order of their increasing atomic numbers. As we move from one element to the next, the number of protons and electrons increases accordingly. This gradual increase in atomic number influences various properties, including the number of valence electrons.
Mercury’s Place in the “d-Block”
Mercury resides in Group 12 of the Periodic Table, known as the “d-block”. This placement indicates that mercury possesses a complete set of d-orbitals in its electron configuration. These d-orbitals are crucial for understanding the chemical reactivity and bonding behavior of mercury.
Valence Electrons: The Gateway to Reactions
Valence electrons are those electrons that reside in the outermost shell of an atom’s electron configuration. They are the key players in chemical reactions, determining an element’s ability to form bonds and interact with other elements.
For mercury, its complete set of d-orbitals and the associated electrons result in a total of two valence electrons in its outermost shell. This unique electron configuration influences mercury’s chemical properties and sets it apart from other elements.
Through our exploration of electron configuration and atomic number, we have gained profound insights into the number of valence electrons in mercury. Its placement in the “d-block” further highlights the interplay between electron configuration and chemical properties. Understanding these concepts empowers us to unravel the mysteries of chemical bonding and reactivity, paving the way for scientific discoveries that will shape our future.