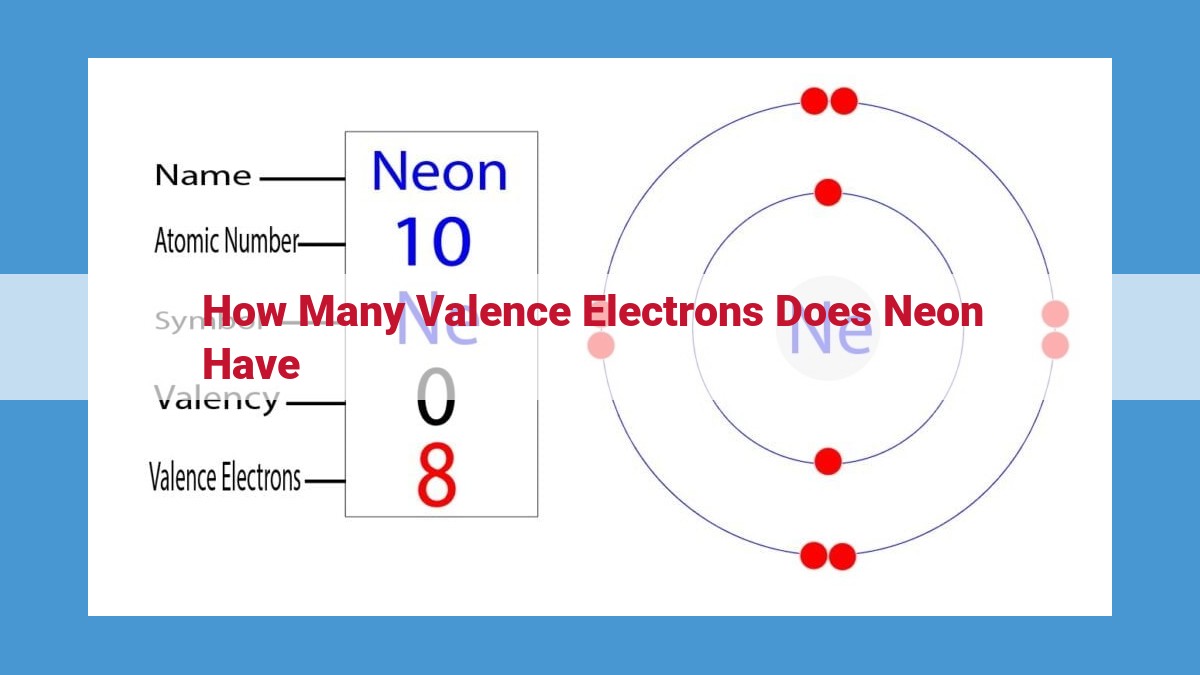
Neon, a noble gas, is renowned for its chemical inertness. Determining the number of valence electrons in neon provides insights into its unreactive nature. Valence electrons, found in the outermost energy level, dictate an atom’s chemical behavior. Neon’s electron configuration (1s² 2s² 2p⁶) reveals eight valence electrons in its 2p subshell. This stable octet configuration, characteristic of noble gases, renders neon unreactive, contributing to its widespread use in lighting and various industrial applications.
Discover the Secrets of Neon’s Valence Electrons
Neon, a noble gas with the symbol Ne, holds an intriguing secret: its valence electrons. These electrons, residing in the outermost energy level of the atom, dictate neon’s chemical reactivity and make it the fascinating element it is. Join us on an adventure as we explore the world of neon’s valence electrons, unraveling their significance and their role in shaping neon’s properties.
What are Valence Electrons?
Valence electrons, the electrons on the outermost energy level of an atom, are the key players in determining an element’s chemical behavior. They are the gatekeepers of reactivity, influencing how an element interacts with other atoms.
The Noble Gas Configuration
Noble gases, like neon, have reached the chemical nirvana of having a full outermost energy level. This stable arrangement grants them an apathy towards chemical reactions, making them inert and reluctant to form bonds with other elements.
Neon’s Electron Configuration
Neon, with an atomic number of 10, boasts the electron configuration 1s² 2s² 2p⁶. The 2p orbital holds six electrons, which are neon’s valence electrons.
Counting Neon’s Valence Electrons
To determine the number of valence electrons in neon, simply tally up the electrons in the outermost energy level (2p orbital). Voilà! Neon has eight valence electrons.
Neon’s Chemical Inertness
The octet rule dictates that atoms with eight valence electrons, like neon, achieve chemical stability. Neon’s stable electron structure, coupled with its full noble gas configuration, explains its low reactivity. It prefers to remain in its solitary state rather than engage in chemical partnerships.
Understanding the number of valence electrons in neon sheds light on its unique chemical properties. Neon’s eight valence electrons and its noble gas configuration bestow upon it a chemical indifference, making it the inert and stable element we know today.
Understanding Valence Electrons:
- Define valence electrons as those involved in chemical reactions.
- Explain how the atomic number determines the number of valence electrons.
Understanding Valence Electrons
In the realm of chemistry, the concept of valence electrons unveils the intriguing secrets of chemical reactions. Imagine valence electrons as the intrepid explorers venturing beyond an atom’s core. These intrepid electrons, residing in the outermost energy level, are the key players in determining the chemical properties of elements.
One crucial factor influencing the number of valence electrons is the atomic number. Each element’s atomic number, a unique identifier assigned to each element on the periodic table, corresponds to the total number of electrons it possesses. Valence electrons, being the outermost electrons, are determined by the atomic number.
For instance, an atom of neon, with an atomic number of 10, has a total of 10 electrons. By virtue of its position in Group 18 (also known as the noble gas group), neon exhibits a stable electron configuration, which means its electrons are arranged in a way that renders it chemically inert.
Determining the Number of Valence Electrons in Neon: A Step-by-Step Guide
Embark on a scientific adventure as we delve into the world of neon and unravel the secrets of its electron structure. Neon, a noble gas, occupies a special place on the periodic table, its atoms exhibiting remarkable stability. In this blog post, we’ll embark on a journey to determine the number of valence electrons in neon, a key factor that governs its chemical behavior.
Noble Gas Configuration: The Secret to Stability
Picture a tranquil haven where electrons reside in harmony. This is the realm of noble gas configurations, where elements like neon find their chemical bliss. The stable electron arrangements of noble gases stem from their fully occupied outermost energy levels. These filled shells create a protective barrier, shielding the atoms from external interactions.
Neon’s Electron Configuration: A Blueprint for Inertness
Let’s focus our attention on the enigmatic neon atom, its electron configuration a blueprint for its chemical properties. Written as 1s² 2s² 2p⁶, this configuration reveals the distribution of electrons across its energy levels. The outermost 2p subshell, highlighted by its superscript ⁶, holds the key to neon’s valence electrons.
Counting Neon’s Valence Electrons: A Numbers Game
To determine neon’s valence electrons, we embark on a mathematical journey. Valence electrons reside in the outermost energy level, so we simply count the electrons occupying the 2p subshell. With its alluring superscript ⁶, this subshell accommodates 6 electrons. Adding to this, the 2s subshell contributes 2 more electrons, bringing the total number of valence electrons in neon to 8.
Significance of Neon’s Valence Electrons: A Tale of Inactivity
Neon’s 8 valence electrons and its noble gas configuration play a pivotal role in its chemical behavior. This stable electron arrangement renders neon chemically inert. Unlike many elements that readily engage in chemical reactions, neon remains aloof, its atoms content in their peaceful electron configuration.
Unveiling the Secrets of Neon’s Electron Structure: A Journey to Valence Electrons
Delve into the intriguing world of chemistry and uncover the mysteries of neon’s electron structure. As we embark on this journey, we’ll explore the realm of valence electrons, unraveling the secrets of neon’s chemical behavior.
Neon: The Inert Noble Gas
Neon, a member of the esteemed Group 18 of the periodic table, holds a special place as a noble gas. With its unwavering stability, it has earned its title as the epitome of chemical inertness. But what makes neon so unreactive? The key lies in its electron configuration.
Valence Electrons: The Gateway to Chemical Reactions
In the world of chemistry, valence electrons play a crucial role in determining an element’s reactivity. These are the electrons that reside in the outermost energy level of an atom, and they are the ones that participate in chemical reactions. The number of valence electrons is dictated by the element’s atomic number, which for neon is 10.
Noble Gas Configuration: A Haven of Stability
Noble gases, including neon, possess a special electron configuration that gives them their exceptional stability. They have a full outermost energy level, meaning all of their valence electrons have found their comfortable homes. This stable arrangement makes them reluctant to gain or lose electrons, contributing to their renowned inertness.
Neon’s Electron Configuration: Unveiling the Secrets
Neon’s electron configuration, written as 1s² 2s² 2p⁶, provides a detailed blueprint of its electron distribution. The first two electrons reside in the innermost 1s orbital, while the next two occupy the 2s orbital. The remaining six electrons, located in the 2p subshell, are neon’s valence electrons.
Determining Neon’s Valence Electrons: Counting the Outermost Electrons
To calculate neon’s valence electrons, we simply tally up the electrons in its outermost energy level, the 2p subshell. With six electrons residing in this subshell, neon boasts a total of eight valence electrons.
The Significance of Neon’s Valence Electrons: Uncovering Chemical Inertness
Neon’s eight valence electrons and its noble gas configuration play a pivotal role in its chemical behavior. The stable arrangement of these electrons makes neon reluctant to participate in chemical reactions. It prefers to maintain its electron balance, preserving its unreactive nature. As a result, neon is widely used in applications requiring an inert gas, such as lighting and high-voltage equipment.
In conclusion, neon’s electron configuration is a testament to its chemical inertness. With eight valence electrons and a stable noble gas configuration, neon stands as a beacon of stability in the realm of chemistry. Understanding electron structure is essential for predicting chemical properties and unraveling the mysteries of the elements.
Calculating Neon’s Valence Electrons:
- Guide the reader through counting the electrons in the outermost energy level (2p orbital).
- Determine that neon has 8 valence electrons.
Determining the Number of Valence Electrons in Neon: A Simple Guide
In the realm of chemistry, understanding electron structure is crucial for predicting the properties of elements. One key aspect of this structure is determining the number of valence electrons, which play a significant role in chemical reactions. Let’s delve into a simple guide to calculating the valence electrons of neon, a noble gas renowned for its exceptional stability.
Understanding Valence Electrons
Valence electrons are the electrons residing in an atom’s outermost energy level. They are the most reactive electrons, actively involved in chemical bonding and determining an element’s chemical properties. The number of valence electrons is directly related to the atomic number of an element, which represents the total number of protons (and electrons) it possesses.
Noble Gas Configuration and Neon’s Electron Structure
Noble gases, including neon, possess a unique electron configuration that contributes to their exceptional stability. These elements have a full outermost energy level, meaning they have eight valence electrons (except for helium, which has two). This stable configuration makes noble gases highly inert and unlikely to react with other elements.
Neon is the tenth element on the periodic table, with an atomic number of 10. Its electron configuration can be represented as 1s² 2s² 2p⁶. This notation indicates that neon has two electrons in its first energy level (1s orbital), two in its second energy level (2s orbital), and six in its third energy level (2p orbital).
Calculating Neon’s Valence Electrons
To determine the number of valence electrons in neon, we count the electrons in its outermost energy level, the 2p orbital. As mentioned earlier, neon has six electrons in this orbital. Therefore, neon has eight valence electrons.
Significance of Neon’s Valence Electrons
Neon’s stable electron configuration, with its eight valence electrons, contributes significantly to its chemical inertness. The presence of this full outermost energy level prevents neon from undergoing chemical reactions, as it does not need to gain or lose electrons to achieve stability. Consequently, neon is a non-reactive gas, widely used in applications such as lighting and cryogenics due to its low reactivity and high thermal conductivity.
Significance of Neon’s Valence Electrons
Neon’s unique electron configuration holds the key to its chemical behavior. With eight valence electrons, neon boasts a noble gas configuration, meaning its outermost energy level is complete. This stable arrangement lends neon its remarkable chemical inertness, making it one of the least reactive elements.
The 2p orbital, where neon’s valence electrons reside, forms a closed shell, satisfying the element’s desire for a stable electron structure. This closed shell shields the inner electrons from external influences, preventing neon from forming chemical bonds with other elements.
Neon’s low reactivity has earned it a place in various applications. Its stable electron structure allows it to be used in specialized lighting systems, including neon signs and fluorescent bulbs. In these applications, neon’s resistance to chemical reactions ensures its longevity and reliability.
The inertness of neon also makes it an ideal candidate for scientific research. Its stable electron configuration provides a reference point for understanding the behavior of more complex elements, allowing scientists to unravel the mysteries of the atomic world.
In conclusion, neon’s eight valence electrons and noble gas configuration bestow upon it an extraordinary chemical property: inertness. This stability makes neon invaluable in various applications, ranging from lighting to scientific research, where its unreactivity and reliability are paramount.