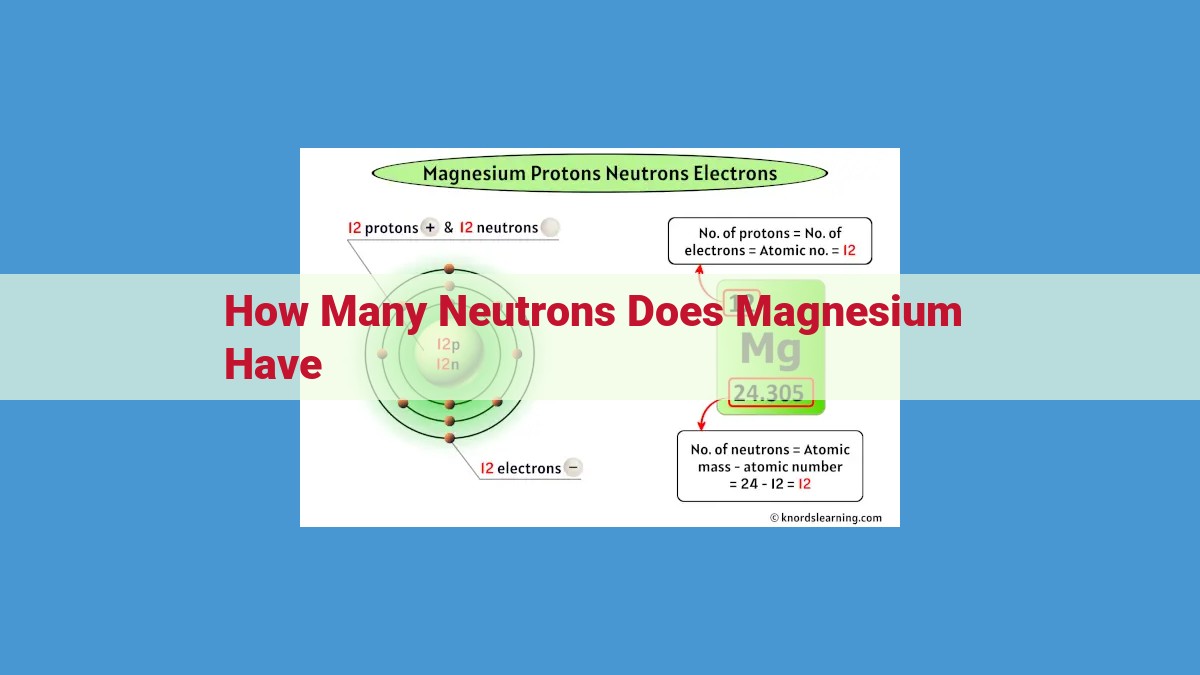
To determine the number of neutrons in magnesium, we explore atomic structure concepts. The atomic number (12) represents the number of protons, which is balanced by the number of electrons. The mass number (24) reflects the total number of protons and neutrons. Subtracting the atomic number from the mass number gives the neutron number, which is 12 for magnesium. Understanding these concepts helps us comprehend the composition and behavior of atoms, including the number of neutrons present in magnesium.
Understanding Atomic Structure: A Journey into the Heart of Matter
Atomic Number: The Key to an Atom’s Identity
Each atom in the vast expanse of the universe possesses a unique fingerprint, a defining characteristic that sets it apart from all others. This identity card is its atomic number, an integer that reveals the number of protons that reside within its nucleus.
Mass Number: A Reflection of Heavyweights and Neutrals
In addition to its protons, an atom also harbors neutrons, enigmatic particles that share the nucleus with their positively charged counterparts. The sum of protons and neutrons gives rise to the mass number, a value that provides a glimpse into the atom’s overall heft.
Electrical Charge: A Balancing Act of Positive and Negative
Atoms strive for a harmonious equilibrium, a delicate balance between positive and negative charges. Protons, with their abundance of positive charges, are counterbalanced by electrons, negatively charged particles that reside in orbits around the nucleus. This intricate interplay ensures that atoms, as a whole, maintain a neutral electrical charge.
Determining the Neutron Number in Magnesium
Understanding the structure of atoms is crucial for unraveling the secrets of the world around us. In this blog post, we’ll embark on a journey to determine the number of neutrons in magnesium, a vital element in biological processes and industrial applications.
Calculating Neutron Number: A Simple Formula
In the heart of every atom lies its nucleus, a dense core that houses protons and neutrons. The number of protons, known as the atomic number, is unique to each element. Magnesium, for instance, has an atomic number of 12, which means it has 12 protons.
The neutron number represents the number of neutrons within the nucleus. Neutrons, devoid of electrical charge, contribute to an atom’s mass without affecting its chemical properties. To determine the neutron number, we employ a simple formula:
Neutron Number = Mass Number – Atomic Number
Applying the Formula to Magnesium
Magnesium’s mass number is 24. This value indicates the total number of protons and neutrons within its nucleus. Substituting the atomic number (12) and mass number (24) into the formula, we get:
Neutron Number = 24 – 12
Neutron Number = 12
Therefore, magnesium has 12 neutrons in its nucleus. This understanding is essential for comprehending its atomic structure and predicting its behavior in various chemical reactions.
Related Concepts for Enhanced Comprehension
- Discuss atomic mass, number of protons, and number of electrons.
- Explain how these concepts relate to the number of neutrons in magnesium.
Understanding the Neutron Number in Magnesium: Unveiling the Secrets of Atomic Structure
Related Concepts for Enhanced Comprehension
In our exploration of the neutron number in magnesium, it’s crucial to grasp the fundamental concepts of atomic structure. Atomic mass represents the combined weight of protons and neutrons within the atom’s nucleus. Number of protons, also known as the atomic number, signifies the positive charge of the nucleus and uniquely identifies each element on the periodic table. On the other hand, the number of electrons orbiting the nucleus determines the atom’s charge and chemical interactions.
Understanding these concepts lays the groundwork for determining the number of neutrons in magnesium. The mass number of magnesium is the sum of protons and neutrons, while the atomic number represents the number of protons. Therefore, by subtracting the atomic number from the mass number, we can determine the number of neutrons.
In the case of magnesium, it has an atomic number of 12, indicating 12 protons in its nucleus. To find the number of neutrons, we consult the periodic table to discover that magnesium’s mass number is 24. By subtracting 12 protons from 24, we arrive at a neutron number of 12.
This exercise highlights the intricate interplay between atomic structure concepts. By comprehending atomic mass, the number of protons, and the number of electrons, we gain a deeper understanding of the elemental composition and properties of atoms, unlocking the secrets of the microscopic realm.