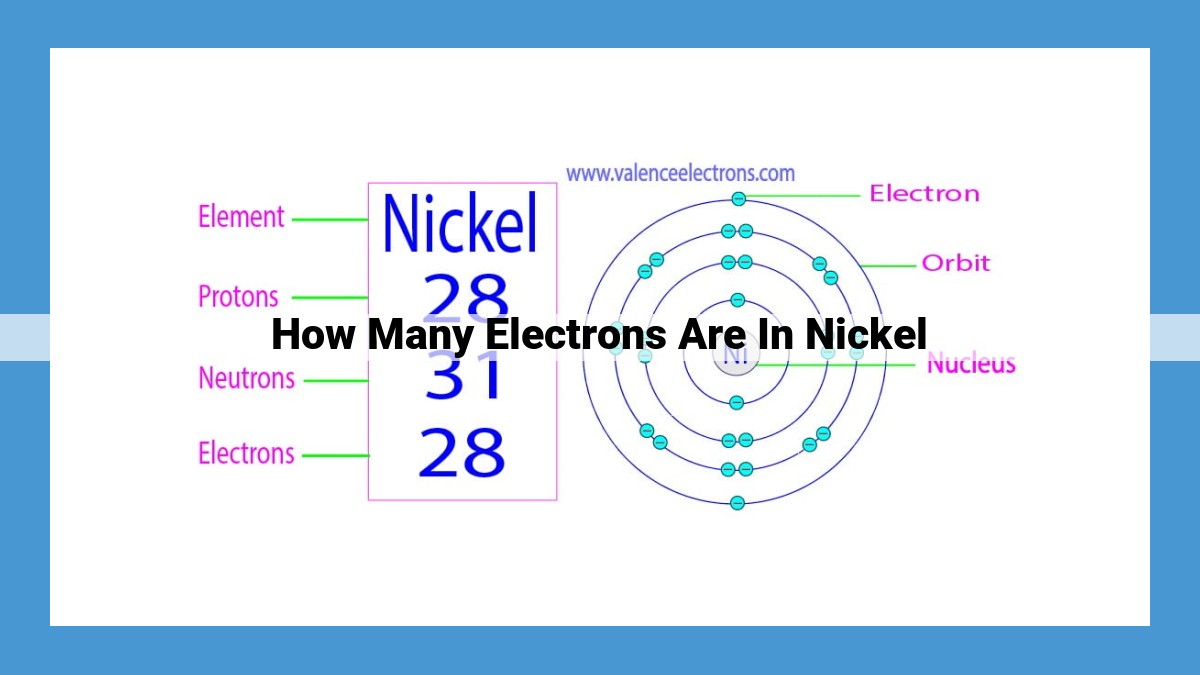
Nickel, with its 28 protons and 28 electrons, plays a crucial role in various industries. To determine its electron count, we delve into atomic structure, where an atom’s electrons occupy specific energy levels or orbitals. Nickel’s electron configuration reveals that it possesses 28 electrons, distributed across four orbitals: 1s (2), 2s (2), 2p (6), 3s (2), 3p (6), 3d (8), and 4s (2). This configuration highlights the presence of 8 valence electrons in the outermost orbitals, influencing nickel’s chemical reactivity by facilitating bond formation. Understanding the number of electrons in nickel not only aids in predicting its chemical behavior but also provides insights into its potential applications in catalysis, corrosion resistance, and other industrial processes.
The Power of Nickel: Unraveling the Secrets of Its Electron Count
In the heart of various industries, nickel emerges as a versatile and indispensable material, shaping our technological advancements and everyday lives. From the gleaming skyscrapers that pierce the sky to the intricate electronics that connect us, nickel plays a pivotal role in countless applications. Its strength, durability, and unique chemical properties make it an essential ingredient in alloys, batteries, and even the production of stainless steel. But what lies at the atomic level that gives nickel its remarkable properties? By embarking on a journey into the realm of quantum mechanics, we will unravel the mystery of the number of electrons that reside within a nickel atom, revealing the secrets behind its chemical behavior.
Diving into the Atom: The Fundamentals of Atomic Structure
At the core of every atom lies a tiny nucleus, a densely packed entity containing protons and neutrons. Protons, with their positive charge, determine an atom’s atomic number, which is a unique identifier for each element. Neutrons, on the other hand, lack any charge and contribute to the atom’s mass. Surrounding the nucleus like a celestial ballet, electrons dance in perpetual motion, occupying specific energy levels known as orbitals. Each orbital can accommodate a maximum of two electrons, and its shape and energy determine the electron’s behavior.
For our enigmatic element, nickel, its atomic number is 28, indicating that it harbors 28 protons within its nucleus. This fundamental property sets the stage for understanding the number of electrons that populate its orbitals.
Unraveling Electron Configuration: A Blueprint of Atomic Architecture
The electron configuration of an atom unveils the precise arrangement of electrons within its orbitals. Beginning with hydrogen, the first element on the periodic table, we can build up the electron configurations of subsequent elements. Each element adds one proton and one electron, progressively filling the orbitals until we reach nickel, our element of interest.
Nickel’s electron configuration can be depicted as: 1s²2s²2p⁶3s²3p⁶3d⁸4s². This cryptic notation reveals a story of electron distribution, providing insights into nickel’s chemical properties. The 1s² portion represents the two electrons in the innermost orbital, closest to the nucleus. The subsequent 2s² and 2p⁶ represent the next two energy levels, each filled to capacity with two and six electrons, respectively.
Delving deeper, we encounter the 3s² and 3p⁶ subshells, mirroring the configuration of the second energy level but now residing at a higher energy. Finally, the 3d⁸ and 4s² subshells complete nickel’s electron configuration, with eight electrons occupying the 3d orbitals and two electrons in the 4s orbital.
Unveiling the Number of Electrons: A Direct Consequence of the Atomic Number
The total number of electrons in an atom is directly proportional to its atomic number. In nickel’s case, the 28 protons in its nucleus dictate the presence of 28 electrons. This fundamental understanding of atomic structure and electron configurations allows us to precisely determine the number of electrons in various elements, providing a crucial foundation for comprehending their chemical behavior.
Unveiling Valence Electrons: The Architects of Chemical Reactivity
Among the electrons that orbit an atom’s nucleus, a special group known as valence electrons plays a pivotal role in chemical reactions. These electrons reside in the outermost orbitals and determine an element’s ability to form chemical bonds with other atoms.
Nickel, with its eight valence electrons (in the 3d and 4s orbitals), is a transition metal renowned for its versatile bonding capabilities. These valence electrons participate in chemical interactions, allowing nickel to connect with a wide range of elements and form diverse compounds with unique properties. The valence electrons act as the architects of chemical reactivity, shaping nickel’s behavior in various applications.
Understanding the number of electrons in a nickel atom is not merely an academic exercise; it holds profound implications for comprehending nickel’s chemical behavior and properties. The electron configuration dictates nickel’s valence electron count, which in turn governs its ability to form chemical bonds. This knowledge empowers scientists and engineers to tailor nickel’s properties for specific applications, unlocking its full potential in industries ranging from aerospace to energy storage.
The journey into the realm of quantum mechanics has unveiled the secrets of nickel’s electron count, illuminating the intricate dance of electrons within its atomic structure. By grasping these fundamental principles, we gain a deeper appreciation for the remarkable properties of nickel and its indispensable role in shaping our technological advancements.
Counting the Electrons in a Nickel Atom: A Journey into the Heart of Matter
In the vast expanse of our universe, there exists an incredible diversity of elements, each with its own unique properties and applications. Among these, nickel stands out as a metal of remarkable importance, playing a vital role in countless industries, from automotive manufacturing to electronics. Understanding the fundamental structure of nickel, and specifically the number of electrons it possesses, is crucial for unlocking its full potential. Embark on a storytelling journey as we unravel this atomic mystery.
At the heart of every atom lies a nucleus, a dense core containing positively charged protons and electrically neutral neutrons. Surrounding the nucleus, like tiny planets orbiting a star, are negatively charged electrons. The atomic number of an element, represented by the symbol Z, indicates the number of protons in its nucleus. In the case of nickel, Z is 28, signifying the presence of 28 protons within its core.
Electrons, on the other hand, exist within specific energy levels or orbitals, which can be visualized as spherical shells around the nucleus. Each orbital has a maximum capacity, dictating the number of electrons it can accommodate. The arrangement of electrons within these orbitals, known as electron configuration, provides insights into an atom’s chemical behavior and properties.
For nickel, its electron configuration is written as: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸. This notation signifies that:
- The innermost orbital, 1s, holds two electrons.
- The subsequent 2s and 2p orbitals each hold two and six electrons, respectively.
- The 3s and 3p orbitals also hold two and six electrons, respectively.
- The outermost 4s orbital holds two electrons.
- The 3d orbital, which is nestled between the 3p and 4s orbitals, holds eight electrons.
By summing the number of electrons in each orbital, we arrive at the total number of electrons in a nickel atom: 28. This number directly corresponds to the atomic number of nickel, demonstrating the fundamental principle that the number of protons in the nucleus is balanced by the number of electrons orbiting it.
Of particular significance are the valence electrons, which reside in the outermost orbitals of an atom and determine its chemical reactivity. In the case of nickel, the outermost 3d and 4s orbitals contain eight valence electrons. These valence electrons play a pivotal role in forming chemical bonds with other atoms, allowing nickel to participate in a wide range of chemical reactions.
Understanding the electron configuration of nickel is not merely an academic exercise; it holds practical implications for various applications. In catalysis, nickel’s eight valence electrons enable it to facilitate chemical reactions by providing a pathway for electron transfer. In metallurgy, nickel’s ability to form strong metallic bonds contributes to its exceptional strength and durability.
As we delve deeper into the world of chemistry and materials science, a comprehensive understanding of electron configurations becomes indispensable. By unraveling the mysteries of atomic structure, we empower ourselves to harness the full potential of elements like nickel, unlocking new frontiers of innovation and shaping our technological landscape.
Define an atom and explain its basic structure
Discover the Secrets of Nickel: Unraveling the Number of Electrons in Its Atomic Realm
Imagine venturing into the microscopic world where atoms, the fundamental building blocks of matter, reside. Among these tiny wonders lies nickel, a versatile element that plays a crucial role in countless industries, from aerospace to construction. To fully grasp nickel’s behavior and applications, we must embark on a quest to determine the number of electrons it possesses, a journey that leads us to the fascinating realm of atomic structure.
Delving into the Realm of the Atom
An atom, the smallest unit of an element that retains its chemical properties, is composed of a dense core called the nucleus and a cloud of electrons orbiting around it. The nucleus, located at the center of the atom, houses two types of subatomic particles: protons and neutrons. Protons carry a positive charge, while neutrons are neutral. The number of protons within the nucleus is known as the atomic number, which is unique for each element. Nickel’s atomic number is 28, indicating that every nickel atom has 28 protons at its core.
Unveiling Nickel’s Electron Configuration
Electrons, negatively charged particles, whirl around the nucleus in designated energy levels or orbitals. Each orbital can hold a specific number of electrons, with the first orbital (closest to the nucleus) holding up to two electrons, the second holding up to eight, and so on. By understanding an element’s electron configuration, we can predict its chemical behavior and reactivity.
Nickel’s electron configuration is 1s²2s²2p⁶3s²3p⁶3d⁸4s². This complex notation depicts the distribution of electrons across its orbitals. The superscript numbers indicate the number of electrons in each orbital, while the letters (s, p, d, f) represent the different types of orbitals. The outermost orbitals, known as valence orbitals, play a significant role in chemical bonding. Nickel has eight valence electrons, occupying the 3d and 4s orbitals, which determine its chemical reactivity and bonding properties.
The Curious Case of Nickel: Unraveling the Mysteries of Its Electron Count
In the realm of industry and technology, nickel stands as a vital element, playing a pivotal role in a myriad of applications. Its versatility extends from the construction of stainless steel to the production of batteries and catalysts. However, to fully grasp the essence of nickel’s behavior and properties, we must delve into the intricate depths of its atomic structure.
Atomic Structure: The Foundation of an Atom
An atom, the fundamental building block of all matter, comprises a nucleus at its core, surrounded by a cloud of whirling subatomic particles called electrons. The nucleus houses positively charged protons and neutral neutrons. The number of protons within an atom’s nucleus determines its atomic number, which uniquely identifies each element.
Nickel, with an atomic number of 28, has 28 protons nestled within its nucleus, establishing its identity as an element distinct from all others.
Electron Configuration: Mapping the Electron Dance
Electrons, the negatively charged inhabitants of the atomic realm, dance around the nucleus in designated energy levels known as orbitals. The arrangement of electrons within these orbitals is termed the electron configuration.
Nickel’s electron configuration, derived from its atomic number, is a story of 28 electrons occupying various orbitals. The first two electrons reside in the innermost 1s orbital, followed by eight electrons in the 2s and 2p orbitals. The 3s and 3p orbitals house six electrons, while the remaining ten electrons distribute themselves within the 3d, 4s, and 4p orbitals.
The Number of Electrons: A Tale of Equalibrium
The total number of electrons in an atom is a delicate balance, always matching the number of protons. This delicate harmony ensures that the atom maintains electrical neutrality, with the positive charges of the protons perfectly offsetting the negative charges of the electrons.
In the case of nickel, its 28 electrons align perfectly with its 28 protons, creating a neutral, stable atom.
Valence Electrons: The Chemical Chameleons
Among the 28 electrons that orbit nickel’s nucleus, the valence electrons stand out as the most influential. These electrons, residing in the outermost orbitals, dictate the chemical reactivity of nickel.
Nickel possesses 8 valence electrons spread across its 3d and 4s orbitals. These electrons, eager to form bonds with other atoms, drive nickel’s diverse chemical interactions, allowing it to play a versatile role in various alloys, compounds, and catalysts.
Our journey into the world of nickel’s electrons has revealed a remarkable story of atomic structure, electron configuration, and chemical reactivity. By unraveling the mysteries of nickel’s electron count, we gain a deeper understanding of its unique properties and pave the way for optimizing its applications in various fields.
Whether in the gleaming facades of buildings or the hidden depths of electronic devices, nickel’s electrons continue to shape our world, their presence a testament to the interconnectedness of matter and the power of scientific exploration.
How Many Electrons Does Nickel Have? Delving into the Heart of an Atom
Nickel, with its versatile properties and wide-ranging applications, has become an indispensable element in countless industries. As scientists delve deeper into the intricacies of its atomic structure, a fundamental question arises: how many electrons reside within a nickel atom?
Electron Configuration: The Blueprint of Atoms
Imagine each atom as a miniature solar system, with a central nucleus harboring protons and neutrons, and electrons orbiting around it like planets. The number of protons in the nucleus, known as the atomic number, determines the element’s identity. Nickel, with an atomic number of 28, possesses an equal number of protons.
Electrons, on the other hand, are arranged in specific energy levels, or orbitals, around the nucleus. These orbitals are depicted using letters (s, p, d, f) and numbers (1, 2, 3, …), with each orbital representing a different energy and shape.
Unraveling Nickel’s Electron Symphony
To determine the number of electrons in nickel, we embark on a journey through its electron configuration. Each orbital has a maximum capacity for electrons: s orbitals hold up to 2 electrons, p orbitals can accommodate up to 6 electrons, while d and f orbitals can hold 10 and 14 electrons, respectively.
Nickel’s electron configuration is [Ar] 3d8 4s2. This enigmatic notation signifies that nickel’s electron configuration mirrors that of argon (Ar), an inert gas with a stable electron arrangement. Beyond this argon-like core, nickel possesses 8 electrons in its 3d orbitals and 2 electrons in its 4s orbital.
Counting the Electrons: The Answer Revealed
The total number of electrons in an atom is equal to its atomic number. Therefore, the number of electrons in nickel is 28, matching its atomic number. This harmonious distribution of electrons explains nickel’s unique chemical behavior and properties.
Valence Electrons: The Key to Chemical Interactions
Among nickel’s electrons, a select group known as valence electrons play a crucial role in chemical bonding. These valence electrons reside in the outermost orbitals, eager to interact with electrons from other atoms. In nickel’s case, its 8 valence electrons (in the 3d and 4s orbitals) govern its ability to form bonds and contribute to its diverse chemical applications.
Understanding electron configurations, including the number of electrons in an atom, empowers scientists and chemists to predict and optimize chemical interactions. By unraveling the secrets of nickel’s atomic structure, we pave the way for its continued use in countless industries, from metallurgy to electroplating and beyond.
Provide nickel’s electron configuration and explain how it is derived from its atomic number
How Many Electrons Does a Nickel Atom Have?
Nickel, a silvery-white transition metal, plays a crucial role in various industries, including steel production, electroplating, and battery manufacturing. Understanding its atomic structure, particularly the number of electrons it possesses, is essential for comprehending its chemical behavior and properties.
Atomic Structure of Nickel
An atom, the fundamental building block of matter, consists of a central nucleus surrounded by orbiting electrons. The nucleus contains positively charged protons and neutral neutrons, while electrons carry negative charges. Each element is distinguished by its atomic number, which represents the number of protons in its nucleus. Nickel’s atomic number is 28, indicating the presence of 28 protons.
Electron Configuration
The electron configuration of an atom describes the arrangement of its electrons within energy levels, known as orbitals. These orbitals can hold a maximum number of electrons, ranging from two to eight. Nickel’s electron configuration is [Ar] 3d8 4s2. This means that it has:
- 18 electrons in its inner shells, which are similar to those of argon (Ar)
- 8 electrons in its 3d orbital
- 2 electrons in its 4s orbital
Number of Electrons
The total number of electrons in an atom equals its atomic number. Since nickel has an atomic number of 28, it has 28 electrons. This is because the number of protons and electrons must be equal to maintain electrical neutrality.
Valence Electrons
Valence electrons are the electrons in the outermost orbitals, which determine an element’s chemical reactivity. Nickel has 8 valence electrons (3d8 and 4s2). These electrons are crucial in forming chemical bonds with other atoms, influencing nickel’s chemical properties.
Understanding the electronic structure of nickel, including the number of electrons it possesses, is paramount for predicting and optimizing its chemical interactions in various applications. Its 28 electrons and 8 valence electrons give nickel unique properties that make it indispensable in industries ranging from steel production to battery manufacturing.
Unveiling the Enigmatic Nickel: A Journey into Its Atomic Architecture
Prologue: Nickel’s Industrial Significance
Nickel, a metal of immense versatility, has found its niche in countless industries. From its role in stainless steel to its presence in batteries, semiconductors, and even coins, nickel plays a pivotal part in shaping our modern world.
Delving into the Heart of an Atom
An atom, the fundamental building block of matter, comprises a tiny, dense nucleus surrounded by a cloud of electrons. Nickel’s atomic number (28) reveals the presence of 28 protons in its nucleus, determining its unique identity among the elements.
Electron Configuration: Unraveling Nickel’s Inner Workings
Electrons, the negatively charged particles orbiting the nucleus, have their own unique arrangement known as electron configuration. Nickel’s configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s².
Each of these alphanumeric notations represents an orbital, a specific region where electrons reside. The capacity of each orbital is denoted by its superscript. For instance, the 3d orbital can accommodate a maximum of 10 electrons.
Determining Nickel’s Electron Count
The total number of electrons in an atom equals its atomic number, so nickel has 28 electrons. This meticulous arrangement of electrons plays a crucial role in understanding the metal’s chemical behavior.
Valence Electrons: The Gateway to Chemical Reactions
Valence electrons, those residing in the outermost orbitals, govern an element’s chemical reactivity. Nickel possesses 8 valence electrons in its 3d and 4s orbitals. These electrons embroil themselves in chemical bonding, forging connections with other atoms.
Understanding nickel’s electron configuration unlocks a treasure trove of information regarding its chemical properties. This knowledge empowers us to harness nickel’s remarkable versatility in a multitude of applications. By unraveling the secrets of electron arrangements, we gain the power to predict and optimize chemical interactions, unlocking the full potential of this enigmatic metal.
Explain that the total number of electrons is equal to the atomic number
How Many Electrons Are in a Nickel Atom? Unlocking the Secrets of an Industrial Powerhouse
In the realm of manufacturing, metallurgy, and energy, nickel stands tall as an indispensable element. From stainless steel to batteries, its versatility drives innovation across countless industries. But hidden within this silvery metal lies a fundamental question: how many electrons reside within its atomic structure?
Atomic Architecture: A Glimpse into Nickel’s Core
Every nickel atom is a minuscule universe unto itself, comprising a tiny nucleus surrounded by orbiting electrons. The nucleus, the heart of the atom, houses protons (positively charged particles) and neutrons (neutral particles). Nickel’s atomic number, 28, signifies the number of protons in its nucleus. In the realm of chemistry, this number dictates the identity and characteristics of any element.
Electron Configuration: Mapping the Electron Dance
Electrons, the negatively charged inhabitants of atoms, populate specific areas called orbitals. These orbitals, like celestial spheres, can hold a maximum number of electrons. In the case of nickel, its electron configuration describes the arrangement of its 28 electrons within these orbitals.
Starting from the innermost orbital, nickel’s electrons fill the 1s, 2s, 2p, 3s, 3p, 4s, and 3d orbitals. Each orbital has a specific capacity: s orbitals can hold a maximum of 2 electrons, while p orbitals can accommodate 6 electrons. The 3d orbital is unique, with the ability to hold up to 10 electrons.
Counting Electrons: A Simple Equation
The total number of electrons in an atom is governed by a fundamental principle: it must always equal the atomic number. For nickel, with an atomic number of 28, this means it possesses 28 electrons. Understanding this simple equation is crucial for unraveling the mysteries of nickel’s chemical behavior.
Valence Electrons: The Key to Chemical Reactivity
Among the 28 electrons in a nickel atom, the outermost ones, known as valence electrons, play a starring role. These electrons, residing in the 3d and 4s orbitals, determine nickel’s ability to form chemical bonds with other elements.
Nickel’s eight valence electrons contribute to its exceptional chemical reactivity. They participate in sharing or transferring electrons, forming bonds that give rise to countless compounds and materials with diverse properties.
Unlocking Nickel’s Potential: A Deeper Understanding
Comprehending the electron configuration of nickel empowers scientists and engineers to tailor its properties for specific applications. By manipulating the number and arrangement of valence electrons, they can engineer nickel-based materials with enhanced strength, corrosion resistance, magnetic properties, and catalytic capabilities.
From alloys and coatings to batteries and catalysts, nickel continues to shape the modern world. Its versatility stems from the fundamental understanding of its electron configuration, unlocking its potential for innovation and progress.
The Enigmatic Nickel: Unveiling the Secrets of Its Inner World
In the realm of industry, nickel shines as a versatile metal, adding strength, toughness, and resistance to countless applications. From towering skyscrapers to sleek electronics, nickel’s presence is omnipresent. To understand its remarkable properties, we embark on a journey to unravel the secrets of its atomic structure, delving into the realm of electrons.
Atomic Anatomy: A Nickel’s Core
Each nickel atom, the fundamental building block of this extraordinary metal, resembles a miniature solar system. At its core lies the nucleus, a dense hub of positively charged protons and uncharged neutrons. Encircling this nucleus like celestial bodies orbit the sun, electrons dance in constant motion.
Nickel’s atomic number, a unique identifier, is 28. This number corresponds to the number of protons within the nucleus, and in the neutral state of the atom, it also determines the number of electrons whirling around it.
Electron Configuration: Mapping the Orbital Landscape
Imagine each electron occupying a specific orbital, like a private residence in a celestial metropolis. These orbitals are arranged in energy levels, with the lowest energy level closest to the nucleus.
Nickel’s electron configuration describes the distribution of its 28 electrons across these orbitals. Starting from the innermost level, 2 electrons reside in the 1s orbital, followed by 8 electrons in the 2s and 2p orbitals. In the third energy level, 15 electrons fill the 3s, 3p, and 3d orbitals. The outermost energy level, the fourth, houses the remaining 2 electrons in the 4s orbital.
Confirming the Electron Count: A Match of Protons and Electrons
The total number of electrons in a neutral atom is equal to its atomic number. In the case of nickel, the atomic number is 28, hence it possesses 28 electrons. This delicate balance ensures the atom’s overall electrical neutrality.
Valence Electrons: The Gateway to Reactivity
Among the 28 electrons orbiting the nickel nucleus, 8 reside in the outermost energy level, the 3d and 4s orbitals. These electrons, aptly termed valence electrons, play a crucial role in nickel’s chemical interactions.
Valence electrons dictate the manner in which nickel atoms bond with other elements, forming alliances that determine its unique properties. Their involvement in chemical reactions makes them pivotal in shaping nickel’s reactivity and behavior.
Our journey into the heart of a nickel atom has illuminated the intricate dance of 28 electrons. This understanding not only unveils the fundamental structure of nickel but also paves the way for predicting and optimizing its chemical properties.
By unraveling the mysteries of electron configurations, we empower ourselves with the knowledge to harness the remarkable versatility of nickel in various fields, from engineering marvels to cutting-edge technologies.
The Curious Case of Nickel’s Electron Count
In the world of materials, nickel stands out as a versatile player, gracing industries from metallurgy to electronics. Understanding the number of electrons in a nickel atom is not only a matter of scientific curiosity but also a key to unlocking its remarkable properties. So, let’s embark on a journey to determine the number of electrons in a nickel atom!
Nickel, like all elements, is made up of atoms. Each atom consists of a central nucleus, housing protons and neutrons, and a surrounding cloud of electrons. Every element has a unique atomic number, which signifies the number of protons in its nucleus. In the case of nickel, its atomic number is 28. This means that a nickel atom has 28 protons, residing safely in its nucleus.
But how does the atomic number relate to the number of electrons? Well, the universe strives for balance, and in an atom, the number of protons has a profound impact on the number of electrons. Every proton carries a positive charge, while electrons bear a negative charge. To maintain electrical neutrality, an atom must have an equal number of protons and electrons.
Now, let’s delve into the enchanting world of electron configuration. This refers to the arrangement of electrons in specific energy levels or orbitals around the nucleus. Each orbital can hold a maximum number of electrons, and nickel’s electron configuration is as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s²
This tells us that nickel has two electrons in the 1s orbital, two in the 2s, six in the 2p, two in the 3s, six in the 3p, eight in the 3d, and two in the 4s orbital.
Valence electrons are the electrons in the outermost energy level, and they play a crucial role in chemical reactions. In nickel’s case, it has eight valence electrons in its 3d and 4s orbitals. These valence electrons are like the social butterflies of chemistry, involved in forming chemical bonds with other atoms, giving nickel its unique properties.
So, to answer the riddle, a nickel atom has 28 electrons. This knowledge opens doors to understanding nickel’s chemical behavior, enabling scientists to tailor its properties for countless applications, from strengthening alloys to powering electronic devices.
The Mystery of Nickel: Unveiling the Secrets of Its Electronic Structure
Nickel, a versatile and ubiquitous metal, plays a pivotal role in countless industries, from aerospace engineering to jewelry making. Its unique properties, such as corrosion resistance and high strength, stem from its intricate atomic structure. In this article, we embark on a journey to unravel the enigma of nickel and determine the number of electrons that inhabit its atomic realm.
Delving into the Atom’s Heart
An atom, the fundamental building block of all matter, consists of a dense nucleus surrounded by a cloud of electrons. The nucleus harbors protons, positively charged particles, and neutrons, neutral particles. Nickel’s atomic number, represented by the symbol Z, is 28. This number governs the number of protons within its nucleus. Protons and electrons balance each other out electrically, making the atom neutral overall.
Mapping the Electron’s Journey
Electron configuration refers to the arrangement of electrons in an atom’s orbitals. Nickel’s electron configuration, derived from its atomic number, reveals a complex tapestry of electrons occupying different energy levels and orbitals.
- 1s²: Two electrons occupy the lowest energy level (n = 1) in the s orbital.
- 2s² 2p⁶: Eight electrons reside in the next energy level (n = 2), with two in the s orbital and six in the p orbitals.
- 3s² 3p⁶ 3d⁸: Ten electrons populate the third energy level (n = 3), with two in the s orbital, six in the p orbitals, and eight in the d orbitals.
- 4s²: The outermost energy level (n = 4) accommodates two electrons in the s orbital.
Counting the Electronic Charge
The total number of electrons in an atom is always equal to its atomic number. Therefore, with an atomic number of 28, nickel possesses 28 electrons.
The Significance of Valence Electrons
Valence electrons, those in the outermost energy level (4s and 3d in the case of nickel), play a crucial role in determining an element’s chemical properties. Nickel has eight valence electrons, making it relatively unreactive compared to elements with more valence electrons.
The valence electrons engage in chemical reactions by forming bonds with other atoms, enabling nickel to participate in a wide range of chemical interactions. This property makes nickel a versatile material for various applications, including electroplating, battery production, and alloying.
Unlocking Nickel’s Chemical Secrets
Nickel’s electron configuration and the number of its valence electrons provide valuable insights into its chemical behavior and properties. By understanding the electronic structure of nickel, scientists and engineers can optimize its use in countless applications, unlocking the full potential of this extraordinary metal.
The Electrical Nature of Nickel: Unveiling the Secrets of Its Chemical Bonds
In the realm of chemistry, understanding the number and arrangement of electrons within an atom holds immense significance. Among the diverse elements, nickel stands out as a versatile metal with applications spanning from batteries to stainless steel. In this article, we embark on a journey to unravel the number of electrons in a nickel atom and delve into the captivating world of electron configurations.
Unveiling the Atomic Structure
Atoms, the fundamental building blocks of matter, consist of a nucleus surrounded by electrons. The nucleus, dwelling at the heart of the atom, harbors protons (positively charged) and neutrons (neutral). Nickel, a transition metal, boasts an atomic number of 28, indicating that its nucleus harbors 28 protons.
The Electron Configuration Puzzle
Electron configuration meticulously describes the spatial arrangement of electrons within an atom’s orbitals. Each orbital can accommodate a maximum of two electrons, adhering to specific energy levels. Nickel’s electron configuration, derived from its atomic number, reveals the distribution of its 28 electrons as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s²
The Significance of Valence Electrons
Among the electron configuration’s intricacies, valence electrons occupy a pivotal position. Residing in the outermost energy levels, valence electrons dictate an atom’s chemical reactivity. _Nickel posee_s 8 valence electrons, occupying its 3d and 4s orbitals. These valence electrons play a crucial role in bonding with other atoms, forming the chemical bonds that govern nickel’s versatile properties.
Chemical Bonding: A Symphony of Electrons
Valence electrons, like social butterflies, seek companionship with other atoms, forming chemical bonds. These bonds arise from the attraction between oppositely charged particles, leading to the stabilization of atoms and the formation of molecules. Nickel’s 8 valence electrons enable it to engage in various bonding scenarios, endowing it with the ability to form alloys, catalyze reactions, and exhibit magnetic properties.
Determining the Number of Electrons in a Nickel Atom: Unlocking the Secrets of a Versatile Metal
Nickel, a lustrous, silver-white metal, plays a pivotal role in a myriad of industries, from aerospace to electronics. Understanding its atomic structure, particularly the number of electrons it holds, is crucial for comprehending its chemical behavior and harnessing its potential.
Atomic Structure
An atom, the fundamental building block of all matter, comprises a nucleus surrounded by electrons. The nucleus consists of protons, positively charged particles, and neutrons, electrically neutral particles. The number of protons in the nucleus, known as the atomic number, defines the element. Nickel, with an atomic number of 28, possesses 28 protons.
Electron Configuration
Electron configuration describes the arrangement of electrons around the nucleus. Each electron occupies a specific energy level or orbital, which can be visualized as concentric shells surrounding the nucleus. Electrons within an orbital are characterized by a set of quantum numbers that define their energy, shape, and orientation.
Nickel’s electron configuration is [Ar] 3d8 4s2. This indicates that it has the same electron configuration as the noble gas argon (Ar) in its inner shells, followed by eight electrons in the 3d orbitals and two electrons in the 4s orbital. The 4s orbital is the outermost orbital and holds the valence electrons, which determine nickel’s chemical reactivity.
Number of Electrons
The total number of electrons in an atom is equal to its atomic number. Since nickel has an atomic number of 28, it has 28 electrons.
Valence Electrons
Valence electrons are the electrons in the outermost orbitals, which are involved in chemical bonding. Nickel has eight valence electrons, two in the 4s orbital and six in the 3d orbital. These valence electrons determine nickel’s ability to react with other atoms and form compounds.
The number of electrons in a nickel atom, as well as its electron configuration, provides valuable insights into its chemical behavior. With 28 electrons, nickel has eight valence electrons that participate in chemical reactions. Understanding these characteristics enables scientists and engineers to harness nickel’s versatility and exploit its potential in various applications.
Relate these findings to nickel’s chemical behavior and properties
Nickel, a versatile metal, plays a crucial role in our modern world, powering batteries in electric vehicles, strengthening alloys in construction, and fighting corrosion in chemical plants. Understanding the number of electrons in a nickel atom is vital for comprehending its chemical behavior and properties. This article delves into the fascinating world of nickel’s atomic structure and reveals the number that governs its interactions.
2. Atomic Structure
At the heart of every nickel atom lies a nucleus, a dense core containing protons and neutrons. The number of protons, known as the atomic number, uniquely defines an element. For nickel, this number is 28, indicating the presence of 28 protons in its nucleus.
3. Electron Configuration
Surrounding the nucleus is a cloud of electrons occupying distinct orbitals. The electron configuration of nickel is 1s²2s²2p⁶3s²3p⁶3d⁸4s². This notation tells us that nickel has eight electrons in its outermost orbitals, namely three in the 3d orbital and five in the 4s orbital.
4. Number of Electrons
The total number of electrons in an atom is always equal to its atomic number. Since nickel has 28 protons, it must also have 28 electrons. This fundamental principle dictates the chemical behavior of nickel.
5. Valence Electrons
The valence electrons are the electrons in an atom’s outermost orbitals, which determine its chemical reactivity. Nickel has eight valence electrons, making it a transition metal. These valence electrons readily participate in chemical bonding, enabling nickel to form strong and versatile alloys with other elements.
6. Nickel’s Chemical Behavior and Properties
Nickel’s electron configuration greatly influences its chemical properties. Its eight valence electrons allow nickel to exhibit a wide range of oxidation states, from +2 to +6. This flexibility makes nickel an essential component in many catalytic processes, such as hydrogenation and oxidation reactions.
Furthermore, nickel’s resistance to corrosion stems from a protective oxide layer that forms on its surface when exposed to air. This layer prevents further oxidation, making nickel an ideal material for applications where corrosion resistance is paramount.
Understanding the number of electrons in a nickel atom is crucial for predicting its chemical behavior and properties. Nickel’s 28 electrons, particularly its eight valence electrons, dictate its versatility in forming alloys, its catalytic activity, and its exceptional corrosion resistance. By comprehending these fundamental properties, scientists and engineers can harness nickel’s unique characteristics in a wide range of industrial and everyday applications.
How Many Electrons Does Nickel Have? A Chemical Adventure
Nickel, a versatile metal, plays a crucial role in industries ranging from coinage to stainless steel production. To fully understand its remarkable properties, it’s essential to delve into the atomic realm and explore the number of electrons it possesses.
Atomic Structure
An atom, the fundamental building block of matter, comprises a nucleus surrounded by electrons. Each element is distinguished by its atomic number, which represents the number of protons in its nucleus. Nickel, with an atomic number of 28, has 28 protons.
Electron Configuration
Electrons occupy distinct energy levels or orbitals around the nucleus. The electron configuration of an element describes the distribution of its electrons in these orbitals. Nickel’s configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s², indicating that it has:
- 1 pair of electrons in the 1s orbital
- 2 pairs of electrons in the 2s and 2p orbitals
- 8 electrons in the 3d orbital
- 2 pairs of electrons in the 3p and 4s orbitals
Number of Electrons
The total number of electrons in an atom is equal to its atomic number. Thus, nickel has 28 electrons. This knowledge plays a pivotal role in determining its chemical properties.
Valence Electrons
Valence electrons are those occupying the outermost energy levels and are responsible for chemical bonding. Nickel has 8 valence electrons in its 3d and 4s orbitals. The number and arrangement of valence electrons dictate an element’s bonding behavior.
Applications of Electron Configuration
Understanding nickel’s electron configuration is vital for predicting and optimizing its chemical interactions. In metallurgy, it helps control the properties of steel alloys by tailoring the number and distribution of valence electrons. In catalysis, nickel-based catalysts are designed with specific electron configurations to enhance their catalytic activity.
Nickel’s unique properties stem from the number of electrons it possesses. By unraveling its electron configuration, scientists and engineers can harness its potential in a diverse range of applications, from engineering alloys to developing efficient energy-saving technologies. Through this atomic-level exploration, we gain insights into the fundamental building blocks of our world and unlock the secrets of chemical interactions that shape our technological advancements.