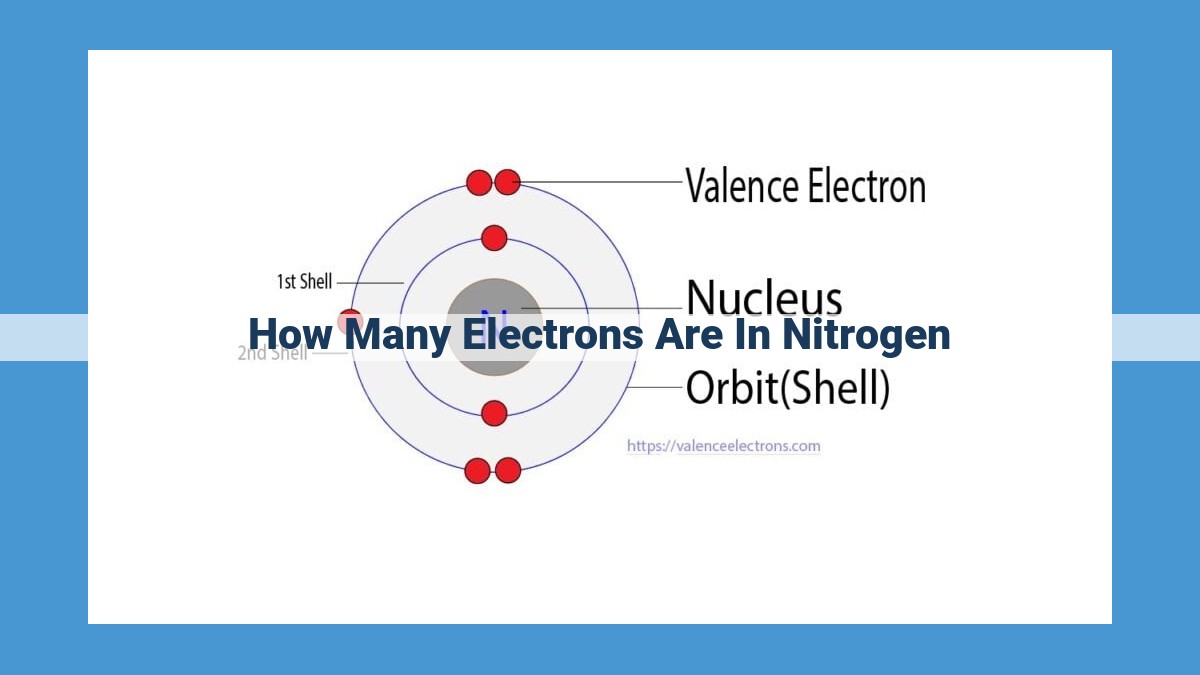
Nitrogen has a total of 7 electrons, determined by its atomic number. These electrons are distributed in two energy levels: the core level with 2 electrons and the valence level with 5 electrons. The valence electrons play a crucial role in nitrogen’s chemical behavior, enabling it to form bonds with other elements and participate in various biological and industrial processes.
Unveiling the Secrets of Electron Configuration: A Journey into the Heart of Nitrogen
In the vast expanse of the atomic realm, each element possesses a unique fingerprint, defined by its electron configuration. For nitrogen, the seventh element on the periodic table, this configuration holds the key to its remarkable chemical properties and diverse applications.
Deciphering the Atomic Architecture
At the heart of every atom lies the nucleus, a tiny powerhouse harboring protons and neutrons. Circling this nucleus, like celestial bodies orbiting a star, are electrons, carrying the negative charge that balances the positive charge of the nucleus.
The number of electrons an atom possesses profoundly influences its chemical properties. This number determines the element’s place on the periodic table and its ability to interact with other elements.
Nitrogen’s Electron Symphony
Focusing our lens on nitrogen, we witness an atom with 7 electrons. These electrons dance around the nucleus, occupying specific energy levels. The innermost energy level, closest to the nucleus, accommodates 2 electrons, referred to as core electrons. These electrons are firmly bound to the nucleus, their energy tightly controlled.
The outermost energy level, furthest from the nucleus, can house up to 8 electrons. This region, known as the valence shell, is home to nitrogen’s 5 valence electrons. These electrons, with their higher energy, play a pivotal role in determining the atom’s chemical reactivity.
The Significance of Valence Electrons
Valence electrons are the gatekeepers of chemical bonding, the process by which atoms unite to form molecules. These electrons participate in the sharing or exchanging of electrons with other atoms, creating strong bonds that hold molecules together.
In the case of nitrogen, its 5 valence electrons endow it with a high degree of chemical activity. This reactivity allows nitrogen to readily combine with other elements, forming a vast array of compounds with diverse properties.
Nitrogen’s Atomic Profile: A Summary
- Number of protons (atomic number): 7
- Number of electrons: 7
- Core electrons: 2
- Valence electrons: 5
Understanding an element’s electron configuration, like deciphering a secret code, unveils the underlying forces that govern its chemical behavior. For nitrogen, its 5 valence electrons hold the key to its versatility and extensive applications in biological processes and industrial chemistry. From the synthesis of fertilizers to the production of explosives, nitrogen’s unique electron configuration has shaped the world around us. By unraveling the secrets of electron configurations, we gain a deeper appreciation for the intricate tapestry of nature’s building blocks.
Valence Electrons: The Powerhouses of Chemical Bonding
In the enchanting world of chemistry, one of the most fascinating concepts lies in understanding the electron configuration of elements. Picture an atom, a minuscule realm comprising a nucleus – a bustling hub of protons and neutrons – and electrons that dance around it like celestial bodies. Among these electrons, a special group known as valence electrons holds the key to an element’s chemical destiny.
Valence electrons, as their name suggests, reside in the outermost energy level of an atom, farthest from the nucleus. As they twirl and spin at the atom’s edge, these adventurous electrons play a pivotal role in determining the element’s chemical reactivity. In other words, valence electrons dictate how eager an element is to cozy up with other elements and form chemical bonds.
Take nitrogen, for instance, an element crucial for life on Earth. With five valence electrons, nitrogen possesses an insatiable curiosity for bonding. These outermost electrons yearn to interact with other atoms, seeking fulfillment in the formation of molecular partnerships. This vibrant chemical nature makes nitrogen an essential player in the symphony of life-sustaining compounds.
The number of valence electrons an element possesses serves as a guiding star, predicting its chemical behavior. Elements with few valence electrons tend to be less reactive, while those with a brimming supply, like nitrogen, are more prone to bonding adventures. Understanding valence electrons is akin to deciphering the secret language of chemistry, empowering us to unravel the intricate dance of chemical reactions.
Core Electrons: The Stable Bedrock of Nitrogen’s Atomic Architecture
In the heart of every nitrogen atom, beneath the bustling surface of valence electrons, lies a stable foundation—the core electrons. These electrons reside in the inner sanctums of the atom, their dance tightly synchronized around the nucleus. Core electrons are the unsung heroes of nitrogen’s atomic structure, providing the stability and foundation upon which nitrogen’s unique chemical properties are built.
Unlike their more energetic counterparts, the valence electrons, core electrons are firmly bound to the nucleus by a relentless electromagnetic force. This tight embrace insulates them from the chemical interactions that shape nitrogen’s reactivity. They remain steadfast in their orbits, unaffected by the chemical skirmishes that rage outside their inner sanctum.
Nitrogen’s atomic core houses two of these stable electrons. These electrons occupy the lowest energy level, closest to the nucleus. Their unwavering presence provides a solid foundation for nitrogen’s chemical behavior, ensuring its stability and preventing it from succumbing to the chaotic forces of electron exchange.
The presence of these core electrons is not merely a matter of atomic architecture; it has profound implications for nitrogen’s chemical destiny. The stability they provide allows nitrogen to engage in a myriad of chemical reactions without compromising its structural integrity. It enables nitrogen to form stable bonds with other elements, creating the building blocks of countless molecules essential for life and industry.
Understanding the role of core electrons in nitrogen’s electron configuration is not just an academic pursuit; it is a key to unraveling the secrets of nitrogen’s chemistry. It provides a deeper appreciation for the intricate dance of electrons within the atom and the profound influence it has on the properties and behavior of the element we know as nitrogen.
Total Electrons: Defining the Element
Understanding the Essence of Nitrogen’s Identity
In the realm of chemistry, an element’s identity is profoundly intertwined with the number of electrons within its atomic structure. Electrons, the negatively charged subatomic particles that orbit the atomic nucleus, play a crucial role in determining the chemical properties and behavior of an element.
For nitrogen, an essential element found in all living organisms and employed in a myriad of industrial applications, the total number of electrons serves as a defining characteristic. The atomic number of an element, a fundamental property that uniquely identifies it, is determined by the number of protons and electrons within its atom. In the case of nitrogen, its atomic number is 7, indicating the presence of 7 protons and, by extension, 7 electrons.
This concept is elegantly encapsulated in the fundamental principle that the total number of electrons in an atom is always equal to its atomic number. Therefore, nitrogen possesses 7 electrons in total, which is a crucial factor in dictating its chemical behavior and its role in the formation of various compounds.