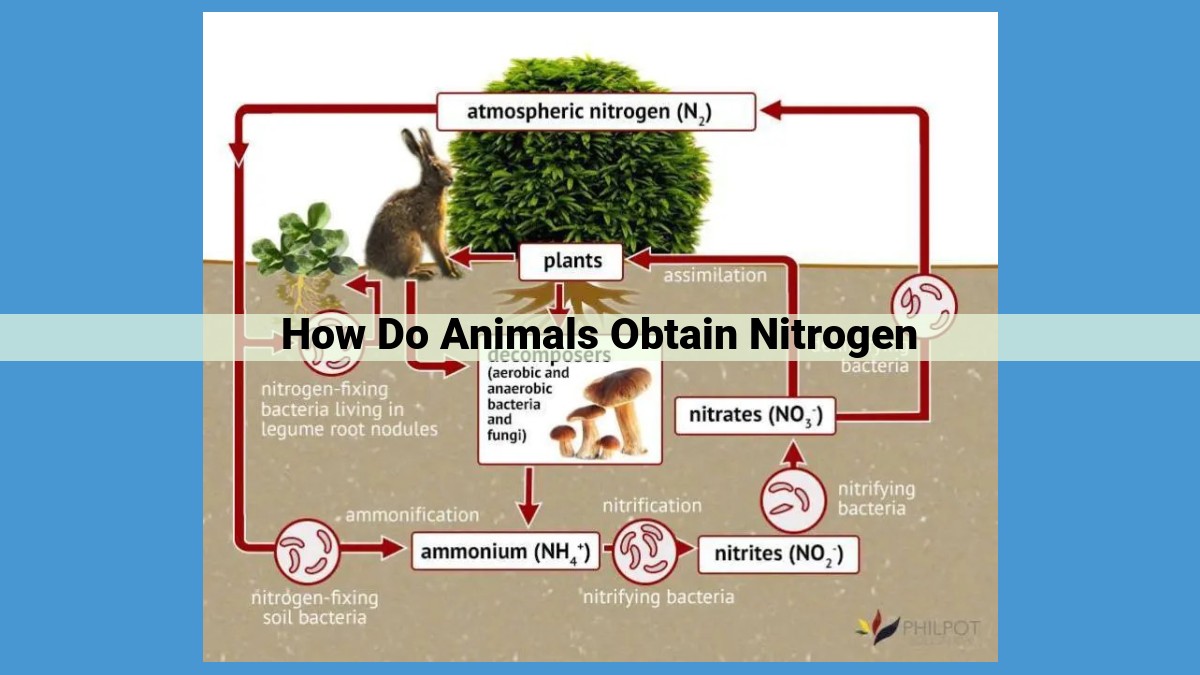
Animals require nitrogen for building essential molecules like proteins and nucleic acids. They acquire nitrogen primarily through food chains which start with nitrogen-fixing bacteria that convert atmospheric nitrogen into biologically available forms. These bacteria form symbiotic relationships with legumes. Through the nitrogen cycle, ammonia is converted to nitrite and then nitrate, which plants absorb and convert into amino acids. Animals consume plants to obtain nitrogen. Excess nitrogen is excreted as waste products, which are decomposed by bacteria, releasing nitrogen back into the environment, completing the cycle.
Nitrogen: The Building Block of Life and the Vital Nitrogen Cycle
Nitrogen is an essential component for the building blocks of life, from proteins to DNA. It forms the very foundation of our existence. However, nitrogen in its pure form is inert and inaccessible to most living organisms. That’s where the nitrogen cycle steps in, playing a crucial role in making nitrogen available to living creatures.
The nitrogen cycle is a continuous process that involves a variety of microorganisms and chemical reactions. It transforms nitrogen into different forms, ensuring a constant supply for life to thrive. The cycle begins with nitrogen fixation, where bacteria, such as Rhizobium, convert atmospheric nitrogen into ammonia, making it usable by plants.
Plants, through photosynthesis, incorporate nitrogen into amino acids and proteins, creating the essential building blocks for their growth and survival. Animals, in turn, consume these nitrogen-rich plant materials, acquiring the necessary nitrogen for their own bodily functions.
Eventually, living organisms excrete nitrogenous waste products, such as ammonia and urea. These compounds return to the environment, where microorganisms break them down through a process called ammonification, releasing ammonia back into the soil.
The nitrogen cycle continues with nitrification, where specialized bacteria convert ammonia to nitrate, a more stable and readily absorbed form of nitrogen. Nitrate is then taken up by plants and utilized for growth.
The beauty of the nitrogen cycle lies in its ability to recycle nitrogen within ecosystems. Through the continuous transformation of nitrogen into different forms, it ensures a constant supply of this vital element for life. However, human activities, such as excessive fertilizer use, can disrupt the delicate balance of the nitrogen cycle, leading to environmental problems.
Understanding and preserving the nitrogen cycle is paramount for our planet’s health and the survival of all living creatures. By embracing sustainable practices, we can safeguard this vital process for generations to come.
The Intriguing Journey of Nitrogen: A Lifeline for Animals
Nitrogen, a fundamental ingredient for life, orchestrates an intricate dance in the environment. It exists in various forms, each playing a vital role in the nitrogen cycle, a seamless process that ensures the availability of nitrogen for all living organisms.
Atmospheric Nitrogen: The Inaccessible Treasure
In its gaseous form, nitrogen is abundant in the Earth’s atmosphere, but it’s largely inaccessible to most organisms. Its triple-bonded molecules, composed of two nitrogen atoms, are extremely stable, making them difficult to break apart and use.
Nitrogen Fixation: Unleashing the Potential
Nature has evolved a remarkable solution to this challenge: nitrogen fixation. Specialized bacteria, such as Rhizobium, act as living catalysts, converting gaseous nitrogen into usable forms, like ammonia. This vital process is often performed in a symbiotic relationship with plants, particularly legumes.
Nitrification: Oxidizing Nitrogen
Once converted to ammonia, nitrogen undergoes another transformation in the soil: nitrification. Beneficial bacteria, including Nitrosomonas and Nitrobacter, oxidize ammonia into nitrite and then nitrate. These ionic forms are easily absorbed and utilized by plants.
Assimilation: Incorporating Nitrogen into Life
Through the power of photosynthesis, plants harness the energy of sunlight to convert inorganic nitrogen into amino acids, the building blocks of proteins. These nitrogen-rich compounds are essential for growth, reproduction, and survival of all living organisms.
Excretion: Releasing Nitrogen
As animals consume plants and other animals, they incorporate nitrogen into their own tissues. However, excess nitrogen must be excreted to maintain a nitrogen balance. Different animals excrete nitrogenous waste in various forms, such as ammonia, urea, and creatine.
Ammonification: Recycling Nitrogen
When plants and animals die, their organic matter is broken down by bacteria and fungi in a process called ammonification. This decomposition releases nitrogen back into the soil as ammonia, completing the nitrogen cycle and replenishing the available nitrogen pool for new life.
Nitrogen Fixation: The Gateway to Life’s Essential Element
Amidst the vast tapestry of life, nitrogen stands as a cornerstone, indispensable for the very fabric of our existence. Its presence in our DNA, proteins, and nucleic acids underscores its pivotal role in the intricate symphony of biological processes. Yet, the acquisition of nitrogen from the air presents a formidable challenge, as it’s largely unavailable in a usable form.
Enter the unsung heroes of the nitrogen cycle: nitrogen-fixing bacteria. These microbial marvels possess the extraordinary ability to convert atmospheric nitrogen into forms that can be assimilated by plants and animals. Among them, Rhizobium bacteria hold a special place.
Imagine a bustling city’s bustling streets, teeming with life and activity. In the realm of the nitrogen cycle, Rhizobium bacteria are the tireless street vendors, transforming inert nitrogen into a valuable resource. This magical transformation takes place within the cozy confines of root nodules, where Rhizobium bacteria establish symbiotic relationships with legumes, such as beans, peas, and clover.
Within these root nodules, a remarkable exchange takes place. Rhizobium bacteria supply the plant with nitrogen compounds, essential for growth and vigor. In return, the plants provide a cozy home and a steady stream of carbohydrates, the energy source that fuels the bacteria’s nitrogen-fixing machinery.
The Nitrogen Cycle: A Vital Partnership Between Legumes and Rhizobium Bacteria
The nitrogen cycle is a complex, yet fundamental process that sustains life on Earth. Nitrogen is an essential element for protein synthesis and plays a crucial role in plant growth and animal development. However, atmospheric nitrogen is not directly usable by most organisms. This is where the symbiotic relationship between legumes and Rhizobium bacteria comes into play.
A Remarkable Alliance
Legumes, such as beans, peas, and clover, possess specialized root structures called root nodules. Within these nodules reside nitrogen-fixing bacteria known as Rhizobium. These microscopic microorganisms have the remarkable ability to convert atmospheric nitrogen into ammonia, a biologically available form of the element.
A Mutually Beneficial Exchange
The relationship between legumes and Rhizobium bacteria is a classic example of mutualism. The plant provides a protective environment for the bacteria within its root nodules, while the bacteria generously fix nitrogen for the plant. This nitrogen can then be used by the legume for its own growth and development.
Significance for Agriculture
The legume-Rhizobium symbiosis is of immense significance for agriculture. Legumes are often used as “green manure” or cover crops to enrich the soil with nitrogen. By planting legumes in rotation with cereal crops, farmers can significantly reduce their reliance on synthetic nitrogen fertilizers.
Benefits of Nitrogen-Rich Soils
Nitrogen-rich soils promote vigorous plant growth, leading to increased crop yields. Nitrogen is a key component of chlorophyll, the pigment that enables plants to harness sunlight for photosynthesis. With ample nitrogen, plants produce more leaves and branches, resulting in higher yields.
Environmental Sustainability
In addition to improving soil fertility, legumes and Rhizobium bacteria contribute to environmental sustainability. By reducing the need for synthetic nitrogen fertilizers, agriculture can minimize water pollution and greenhouse gas emissions associated with fertilizer production.
The symbiotic relationship between legumes and Rhizobium bacteria is a remarkable example of nature’s ingenuity. This alliance plays a vital role in the nitrogen cycle, ensuring the availability of nitrogen for plant growth and animal survival. By harnessing this natural partnership, agriculture can benefit from increased crop yields, soil health, and environmental preservation.
Balancing the Nitrogen Cycle: The Role of Denitrification
In the intricate tapestry of the nitrogen cycle, a crucial process known as denitrification plays a delicate balancing act. While nitrogen fixation captures atmospheric nitrogen, denitrification ensures it doesn’t accumulate excessively in the ecosystem.
Denitrifying Bacteria: Nature’s Nitrogen Removers
Denitrifying bacteria are the unsung heroes of the nitrogen cycle. These microorganisms possess a remarkable ability: they can “breathe” nitrate (NO3-) instead of oxygen (O2). This unique metabolism allows them to convert nitrate into nitrogen gas (N2), releasing it back into the atmosphere.
Balancing the Nitrogen Equation
Denitrification acts as a safety valve in the nitrogen cycle. When nitrogen fixation rates soar, leading to an excess of nitrate, denitrifying bacteria step up to restore equilibrium. They “clean up” the excess nitrate, preventing it from accumulating and causing environmental problems.
Implications for Agriculture and the Environment
Denitrification plays a critical role in agriculture. Excessive nitrate levels in soils can harm crops by inhibiting root growth and reducing yields. By promoting denitrification, farmers can help prevent nitrate buildup and maintain soil fertility.
Denitrification also protects water bodies from nitrate pollution. High nitrate levels in lakes and rivers can lead to eutrophication, a condition that causes excessive algae growth and can suffocate aquatic life. Denitrification helps safeguard water quality by reducing nitrate concentrations.
Denitrification ensures the sustainable cycling of nitrogen, maintaining a delicate balance that supports life on Earth. This process prevents nitrogen overload, protects ecosystems, and contributes to agricultural productivity. Understanding and preserving the intricate workings of the nitrogen cycle, including the crucial role of denitrification, is essential for ensuring the health of our planet for generations to come.
The Nitrogen Cycle: Unveiling the Secrets of Animal Nitrogen Acquisition
Life as we know it wouldn’t be possible without nitrogen, the indispensable element that forms the building blocks of proteins, DNA, and even the chlorophyll that powers photosynthesis. Understanding the nitrogen cycle is crucial to unraveling how animals acquire this essential nutrient.
Nitrification: A Vital Step in Nitrogen’s Transformation
As part of the nitrogen cycle, the conversion of ammonia into nitrate is a key step in making nitrogen available to plants and, by extension, animals. This transformation involves two remarkable bacteria: Nitrosomonas and Nitrobacter.
Nitrosomonas, the first in this microbial duo, takes ammonia excreted by animals and oxidizes it to nitrite. This reaction releases energy, which Nitrosomonas uses to synthesize its own food.
The newly formed nitrite is then taken up by Nitrobacter, which oxidizes it further into nitrate. Nitrate is a stable and mobile form of nitrogen that can be readily absorbed by plants.
The Significance of Nitrate
Nitrate is the primary form of nitrogen used by plants. It’s easily absorbed by plant roots, where it’s utilized in the synthesis of amino acids, proteins, and chlorophyll. Chlorophyll, the green pigment essential for photosynthesis, contains a magnesium atom surrounded by a nitrogen-containing porphyrin ring.
The Nitrogen Cycle: A Delicate Balance
The nitrogen cycle is a continuous process that ensures a steady supply of nitrogen for life on Earth. However, human activities, such as excessive fertilizer use, can disrupt this delicate balance. Understanding the intricacies of the nitrogen cycle is crucial for preserving our planet’s environmental sustainability and ensuring the well-being of future generations.
Nitrate: The Vital Nutrient for Animal Growth and Survival
In the intricate web of life, nitrogen plays a fundamental role as a building block of the essential molecules that sustain all living organisms. Nitrate, a stable and easily absorbed form of nitrogen, is a veritable treasure for animals.
As the centerpiece of the nitrogen cycle, nitrate emerges as the nutrient that enables animals to thrive. Unlike atmospheric nitrogen, which is inaccessible to most organisms, nitrate’s unique chemistry makes it readily available for uptake. Through the process of nitrification, industrious bacteria such as Nitrosomonas and Nitrobacter diligently transform ammonia, the initial product of nitrogen fixation, into nitrate.
Nitrate’s importance stems from its stability and solubility. Unlike ammonia, which can volatilize and escape into the atmosphere, nitrate remains in the soil or water, ensuring a steady supply for plants and animals. Its solubility allows for efficient absorption through roots or digestive tracts, making it an ideal nutrient for plant and animal growth.
In plants, nitrate serves as the primary source of nitrogen for synthesizing amino acids, the building blocks of proteins. Without nitrate, plants would struggle to produce the essential proteins needed for growth, reproduction, and disease resistance. Animals, in turn, rely on plants as their primary source of nitrogen. They consume plants or other animals to obtain the amino acids and proteins they need for building tissues, repairing cells, and producing hormones.
In the animal kingdom, nitrate’s importance extends beyond its role in protein synthesis. It also plays a crucial role in the production of nucleic acids, the molecules that carry genetic information. Without nitrate, animals would be unable to produce DNA and RNA, the building blocks of life.
The availability of nitrate in the environment is essential for animal survival. Fertilizer use in agriculture has significantly increased nitrate levels in many ecosystems, benefiting both crops and the animals that depend on them. However, excessive nitrate levels can also pose challenges, such as water contamination and potential health risks to animals.
Therefore, understanding the delicate balance of the nitrogen cycle and the role of nitrate as a vital nutrient is crucial for maintaining healthy ecosystems and ensuring the well-being of animals. By respecting the delicate equilibrium of nature, we can safeguard the availability of this essential nutrient for generations to come.
The Nitrogen Cycle and Animal Survival: Exploring the Journey of Nitrogen Through the Environment
Embark on a captivating journey through the fascinating world of the nitrogen cycle, an intricate dance of life, death, and transformation. Nitrogen, an essential building block for proteins, nucleic acids, and chlorophyll, is a fundamental component of all living organisms. Understanding its intricate journey through the environment is key to appreciating the harmony and delicate balance of our planet.
Animal Nitrogen Acquisition: The Source of Ammonia
Animals acquire nitrogen primarily through the food they consume. As they metabolize ingested proteins, they release ammonia as a waste byproduct. This ammonia serves as a critical source of nitrogen for the nitrogen cycle, initiating a remarkable chain of transformations.
Animal excretion is a crucial step in the release of ammonia into the environment. Mammals primarily excrete urea, an organic compound containing two ammonia molecules. Birds and reptiles, on the other hand, excrete uric acid, a more concentrated form of nitrogen waste. Aquatic animals, such as fish, excrete ammonia directly into the water.
The release of these nitrogen-rich compounds provides the foundation for the nitrogen cycle, ensuring a continuous supply of this vital nutrient throughout ecosystems. From animal excretions to the decomposition of organic matter, the journey of nitrogen unfolds, shaping the intricate web of life on Earth.
The Role of Photosynthesis and Nitrogen in Plant Growth
Photosynthesis: Capturing the Sun’s Energy
In the intricate web of life on Earth, plants hold a pivotal role as the foundation of food chains. Their ability to harness the sun’s energy through photosynthesis drives the entire ecosystem. During this remarkable process, plants absorb sunlight, water, and carbon dioxide from the atmosphere.
Nitrogen: Essential for Amino Acids and Proteins
Nitrogen, a crucial element for all living organisms, plays a particularly vital role in plant growth. It is an indispensable component of amino acids, the building blocks of proteins. Proteins are essential for a wide range of plant functions, including growth, development, and reproduction.
Photosynthesis and Nitrogen Utilization
Within plant cells, photosynthesis provides the energy needed to convert inorganic nitrogen (such as nitrate or ammonium) into organic forms. This conversion process involves the incorporation of nitrogen into amino acids and eventually into proteins.
Chlorophyll: The Catalyst for Nitrogen Absorption
The green pigment chlorophyll plays a central role in photosynthesis. It absorbs sunlight and initiates a chain of reactions that ultimately reduce nitrogen (convert it from a more oxidized to a less oxidized state) and incorporate it into organic compounds.
Nitrogen-Containing Compounds in Plants
The nitrogen acquired through photosynthesis is utilized by plants to synthesize a range of nitrogen-containing compounds, including:
- Amino acids (e.g., glycine, valine, phenylalanine)
- Proteins (e.g., enzymes, hormones, structural proteins)
- Nucleic acids (e.g., DNA, RNA)
- Chlorophyll
These compounds are essential for plant growth, development, and cellular function. Their presence ensures the robust health and vitality of plant life, which in turn sustains the entire food chain and the delicate balance of the Earth’s ecosystems.
Chlorophyll: The Green Key to Transforming Inorganic Nitrogen
In the ever-spinning dance of life, nitrogen plays a central role as the building block of proteins, nucleic acids, and other essential molecules. But how does this vital element, abundant in the atmosphere, become accessible to living organisms? Enter chlorophyll, the green pigment found in plants, the maestro of the nitrogen cycle.
Without chlorophyll, plants would be mere spectators in the nitrogen game. This remarkable pigment, nestled within chloroplasts, has a unique ability to capture the energy of sunlight and use it to convert inorganic nitrogen compounds, like nitrate and ammonium, into organic forms.
Through a series of intricate chemical reactions, chlorophyll orchestrates the formation of amino acids, the basic units of proteins. These amino acids are then assembled like Lego blocks to create the complex proteins that are essential for every aspect of plant growth and survival.
In essence, chlorophyll acts as a gateway, converting unusable inorganic nitrogen into the organic forms that plants can utilize to craft the building blocks of life. It’s a vital process that supports not only plant survival but also the entire food chain that depends on it.
So, the next time you admire the vibrant green hues of nature, remember that you’re witnessing the silent yet extraordinary work of chlorophyll, the unsung hero of the nitrogen cycle and the foundation of terrestrial life.
Assimilation: Incorporating Nitrogen into Organic Matter
Nitrogen, the foundation of life, plays a crucial role in constructing the essential building blocks of living organisms. As plants go through the remarkable process of photosynthesis, they harness sunlight’s energy and use it to convert inorganic nitrogen into the organic forms that animals and all other life require.
In plants, nitrogen is a vital component of chlorophyll, the green pigment that enables photosynthesis. Chlorophyll traps light energy and uses it to combine nitrogen with other elements to produce amino acids. These amino acids are then assembled into proteins—the fundamental building blocks of life.
Animals, unable to synthesize their own amino acids, rely on plants for nitrogen. When animals consume plants, they ingest complex nitrogen-containing compounds that their bodies break down into simpler forms. These simpler forms can then be used to build animal proteins and other essential compounds.
Examples of Nitrogen-Containing Compounds in Plants:
- Amino acids (e.g., glycine, glutamine, asparagine)
- Proteins (e.g., enzymes, structural proteins)
- Nucleic acids (DNA, RNA)
- Chlorophyll
Examples of Nitrogen-Containing Compounds in Animals:
- Proteins (e.g., muscle proteins, digestive enzymes)
- Nucleic acids (DNA, RNA)
- Creatine (an energy storage molecule in muscles)
- Urea (a waste product excreted by many animals)
Animal Excretion: Releasing Nitrogen Compounds
Animals play a crucial role in the nitrogen cycle by releasing nitrogen waste products into the environment. These compounds include:
-
Ammonia: This is a toxic waste product that is primarily excreted by aquatic animals and certain amphibians. It is highly concentrated and can be harmful to organisms if not quickly diluted.
-
Urea: This is a less toxic waste product that is excreted by most terrestrial animals, including humans. It is less concentrated than ammonia and is less harmful to organisms.
-
Creatine: This is a waste product that is produced when muscle tissue breaks down. It is excreted by animals that have a high level of muscle mass.
Excretion and Nitrogen Balance
Excretion is an essential process for animals to maintain nitrogen balance. Nitrogen is a critical element for building proteins and other essential molecules. However, excess nitrogen in the body can be toxic. Excretion allows animals to remove excess nitrogen and keep their nitrogen levels within a healthy range.
Nitrogen in the Environment
The nitrogen compounds that animals excrete are returned to the environment, where they can be used by other organisms. For example, ammonia can be converted into nitrate by bacteria, which is then used by plants to make proteins. Urea can also be converted into nitrate by bacteria, or it can be broken down by other microorganisms to produce ammonia. Creatine is broken down by microorganisms to produce ammonia and other waste products.
The release of nitrogenous waste products by animals is an important part of the nitrogen cycle, which is essential for the survival of all living organisms.
The Role of Nitrogenous Waste Products in Maintaining Nitrogen Balance
Nitrogenous waste products play a crucial role in maintaining nitrogen balance within organisms. These compounds help regulate the body’s nitrogen levels, ensuring that animals have the necessary nitrogen for body functions, such as protein synthesis and energy production.
Ammonia is one of the primary nitrogenous waste products excreted by animals. It is produced when amino acids are broken down in cells. Ammonia is highly toxic, and animals must convert it to less harmful forms before excreting it.
Urea is the main nitrogenous waste product excreted by mammals. It is formed when ammonia is combined with carbon dioxide in the liver. Urea is less toxic than ammonia and can be easily excreted in urine.
Creatine is a nitrogen-containing compound found in muscle tissue. When muscle tissue is broken down, creatine is released and excreted in urine. Creatine excretion helps maintain nitrogen balance in animals.
These nitrogenous waste products are essential for maintaining nitrogen balance within organisms. They allow animals to regulate their nitrogen levels and ensure that they have the necessary nitrogen for body functions. Without these compounds, animals would quickly become nitrogen deficient and could not survive.
The Nitrogen Cycle: Animal Nitrogen Acquisition and Excretion
Nitrogen is a crucial element for all life forms, yet it exists in an atmospheric form that most organisms cannot use directly. Enter the nitrogen cycle, a complex natural process that converts atmospheric nitrogen into usable forms for plants and animals.
One critical step in the nitrogen cycle is excretion, the process by which animals release nitrogenous waste products back into the environment. Excretion serves as a vital mechanism for maintaining nitrogen balance within organisms and plays a significant role in the recycling of nitrogen within ecosystems.
Ammonia and Urea: Key Nitrogenous Waste Products
Animals excrete nitrogenous waste products in various forms, depending on their species and metabolic pathways. Ammonia is a common waste product in aquatic animals and some terrestrial animals, while urea is the primary waste product in humans and other mammals. Creatine, another nitrogen-containing compound, is excreted by mammals as well.
The Importance of Excretion in Nitrogen Recycling
When animals excrete nitrogenous waste products, they are essentially releasing nitrogen back into the environment. This nitrogen can then be utilized by other organisms, completing the nitrogen cycle. Without excretion, nitrogen would accumulate within animal bodies, leading to toxic levels and ultimately impairing their health.
Furthermore, the nitrogen released through excretion serves as a valuable resource for plants. Plants absorb nitrogen from the soil, using it to synthesize proteins and other essential compounds. This nitrogen uptake supports plant growth and productivity, which, in turn, provides sustenance for animals and humans.
In summary, excretion is a crucial step in the nitrogen cycle, enabling animals to maintain nitrogen balance while simultaneously providing nitrogen to plants. This intricate process ensures the continuous availability of nitrogen for life on Earth.
Ammonification: Nature’s Decomposers
In the ever-evolving symphony of life, decomposition plays an orchestral role, breaking down organic matter to recycle essential elements like nitrogen. And in this intricate dance, bacteria and fungi are the unsung heroes, transforming complex compounds into simpler, more accessible forms.
Bacteria, with their micro-sized prowess, use their digestive arsenal of enzymes to break down organic nitrogen compounds, such as proteins and nucleic acids. They release simpler molecules, including ammonia, into the environment, enriching the soil or aquatic ecosystems for other organisms to utilize.
Fungi, on the other hand, employ their mysterious network of hyphae to search for and absorb organic matter. These filaments secrete enzymes that break down complex nitrogen-containing molecules into smaller, usable forms, ultimately releasing ammonia into the environment.
This process of ammonification is fundamental to the nitrogen cycle, as it converts organic nitrogen back into a usable form for plants and other organisms. Without it, nitrogen would be trapped in organic matter, unavailable for life to thrive.
Moreover, ammonification plays a vital role in the decomposition of dead organisms, releasing essential nutrients back into the soil or water. These nutrients can then be absorbed by plants, continuing the cycle of life and renewal. So, as we appreciate the stunning beauty of nature’s tapestry, let us not forget the microscopic heroes beneath our feet, who diligently perform their hidden symphony of decomposition, ensuring the health and vitality of our planet.
Describe the conversion of organic nitrogen into ammonia by microorganisms.
Ammonification: Releasing Nitrogen’s Hidden Energy
As organic life meets its inevitable end, the symphony of nature’s recycling begins. Microorganisms, like skilled chemists, step onto the stage, their task: to transform the organic nitrogen locked within once-living beings into ammonia, a vital nutrient for life’s eternal dance.
These microbial maestros, primarily bacteria and fungi, possess the uncanny ability to decompose complex organic compounds, breaking them down into their simpler components. The key to this transformative process lies in their ability to liberate ammonia (NH3) from organic matter.
Imagine these microorganisms as nature’s architects, patiently dismantling the intricate structures of proteins and nucleic acids. As they toil, they release ammonia, a molecule that serves as a treasure chest of nitrogen. This precious substance becomes the building block for a myriad of other nitrogen-containing compounds, essential for the very fabric of life.
The conversion of organic nitrogen into ammonia by microorganisms is a rhythmic process, akin to a symphony. Each microbe plays its part, its enzymes acting as the instruments of transformation. Together, they orchestrate the release of ammonia, a vital nutrient that will nourish plants, enrich the soil, and sustain the delicate web of life.
Emphasize the importance of ammonification in recycling nitrogen within the ecosystem.
Ammonification: Nature’s Nitrogen Recycling Center
In the captivating tapestry of life, nitrogen plays an indispensable role. As a fundamental building block of proteins and nucleic acids, it weaves the very fabric of organisms. However, capturing nitrogen from the vast atmosphere poses a formidable challenge, turning the nitrogen cycle into a complex dance of conversions and transformations.
One crucial step in this cycle is ammonification. This process not decomposes but rather recycles. As organic matter—once vibrant creatures or fallen leaves—surrenders to the inevitable embrace of decay, an army of invisible microorganisms, including bacteria and fungi, swoops in to break down these complex compounds. With their enzymatic prowess, they release ammonia, a simpler form of nitrogen.
Ammonification, though often shrouded in the shadows of more glamorous nitrogen cycle processes, is a vital cog in nature’s nitrogen machine. It ensures a steady supply of ammonium, the first step in converting atmospheric nitrogen into forms accessible to plants.
Imagine a lush rainforest, where vibrant plants tower towards the sky like emerald cathedrals. Deep within the soil, teeming with life, microorganisms labor tirelessly to break down decaying plant matter. The resulting ammonia rises through the soil, absorbed by plant roots thirsty for nitrogen. This nitrogen nourishes the plants, enabling them to flourish and produce the fruits and foliage that sustain the entire ecosystem.
Without ammonification, the nitrogen cycle would falter. Nitrogen would remain trapped in decaying organic matter, unavailable to plants and the entire food chain. Ammonification, therefore, serves as a hidden but indispensable force, keeping the wheels of life turning.
The Nitrogen Cycle and Animal Survival: A Vital Connection
In the tapestry of life, nitrogen stands as an indispensable element, the foundation upon which all organisms thrive. This vital element is constantly cycled through the living world, a continuous journey that ensures its availability for animals and plants alike.
Nitrogen Fixation: Capturing the Life-Giving Gas
At the heart of the nitrogen cycle lies nitrogen fixation, a remarkable process by which certain bacteria, like Rhizobium, draw nitrogen from the air and convert it into forms that plants can absorb. This partnership between bacteria and legumes, such as beans and peas, enables plants to flourish in nitrogen-poor soils.
Nitrification: Transforming Ammonia to Nitrate
Once nitrogen enters the soil, bacteria like Nitrosomonas and Nitrobacter take over. They convert ammonia, a nitrogen-containing waste product of animals, into nitrate, a stable and easily absorbed form of nitrogen. This process, known as nitrification, is crucial for plants to fully utilize the nitrogen available to them.
Assimilation: Building Blocks of Life
Plants, the primary producers in the ecosystem, utilize nitrate to synthesize amino acids and proteins, the essential building blocks of life. This process, powered by photosynthesis, transforms inorganic nitrogen into organic compounds, making it available for animals to consume.
Excretion: Releasing Nitrogen Back into the Environment
Animals play a vital role in the nitrogen cycle by excreting nitrogenous waste products, such as ammonia and urea. These compounds are then broken down by bacteria and fungi through a process called ammonification, which releases ammonia back into the soil.
Ammonification: Recycling Nitrogen
Bacteria and fungi are the unsung heroes of the nitrogen cycle. They decompose organic matter, releasing ammonia into the soil. This ammonia can then be used by nitrifying bacteria to convert into nitrate, completing the cycle and replenishing the available nitrogen for plants.
The nitrogen cycle is an intricate dance of life, an ongoing process that ensures the availability of this essential element for all organisms. It highlights the interconnectedness of the living world and the vital role each species plays in maintaining the delicate balance of our planet’s ecosystems.
The Nitrogen Cycle and Animal Survival: Human Impact on Nature’s Balancing Act
In the delicate ecosystem of our planet, the nitrogen cycle plays a crucial role, ensuring the availability of nitrogen, an essential element for all life. However, human activities, particularly the excessive use of fertilizers, have the potential to disrupt this intricate dance of nature.
One of the key steps in the nitrogen cycle is nitrogen fixation, the conversion of atmospheric nitrogen into usable forms by nitrogen-fixing bacteria. This process is essential for replenishing the nitrogen pool in the soil, which plants rely on for growth. Fertilizer use can augment nitrogen fixation rates, but it also comes with consequences.
Excess nitrogen from fertilizers can lead to eutrophication, a condition where nutrient overload causes algal blooms, depleting oxygen levels and harming aquatic life. Runoff from fertilized fields also contributes to nitrate contamination of groundwater, posing health risks and affecting drinking water quality.
Furthermore, the increased nitrogen levels in the soil can disrupt the delicate balance of the nitrogen cycle. It can inhibit the natural process of ammonification, the breakdown of organic matter by microorganisms, releasing ammonia into the environment. This disruption can hinder the availability of nitrogen for plants and affect the overall productivity of ecosystems.
The consequences of human activities on the nitrogen cycle underscore the need for responsible fertilizer use and sustainable agricultural practices. By understanding the delicate balance of nature, we can ensure the preservation of the nitrogen cycle for the well-being of our planet and its inhabitants.
The Delicate Dance of the Nitrogen Cycle: Preserving Environmental Sustainability
In the tapestry of life, nitrogen plays an indispensable role. It weaves its way into the very fabric of our existence, from the proteins that build our bodies to the chlorophyll that nourishes our planet. The nitrogen cycle, a ceaseless dance between nature’s elements, ensures the availability of this essential nutrient. Yet, like a fragile ballerina, the nitrogen cycle faces challenges from human activities that threaten its delicate equilibrium.
The nitrogen cycle begins with nitrogen-fixing bacteria, invisible heroes that capture atmospheric nitrogen and transform it into a form usable by plants. Legumes, those humble plants with their root nodules, foster a symbiotic alliance with these bacteria, accessing the precious nitrogen they need to thrive. However, the cycle is not a one-way street. Denitrifying bacteria, the unsung villains, break down nitrates back into atmospheric nitrogen, completing the circuit.
As plants photosynthesize, they harness nitrogen to create amino acids and proteins, the building blocks of life. Animals, in turn, consume these plants, incorporating nitrogen into their own tissues. When animals excrete waste products, such as ammonia and urea, they release nitrogen back into the environment. This waste forms the foundation for another vital step in the cycle: ammonification. Microorganisms decompose this organic matter, producing ammonia, which then undergoes nitrification, transforming into nitrate.
The nitrogen cycle is a continuous loop, essential for the health of our planet. However, human activities, particularly the overuse of fertilizers, can disrupt this delicate balance. Excessive nitrogen runoff from farms pollutes waterways, creating algal blooms that deplete oxygen from the water, endangering aquatic life. It also contributes to the creation of nitrous oxide, a potent greenhouse gas.
Understanding the intricacies of the nitrogen cycle is paramount for safeguarding our environmental future. It requires a collaborative effort, from farmers adopting sustainable practices to individuals making informed choices about their consumption. By preserving the delicate dance of the nitrogen cycle, we ensure the long-term health of our planet and the well-being of all who call it home.