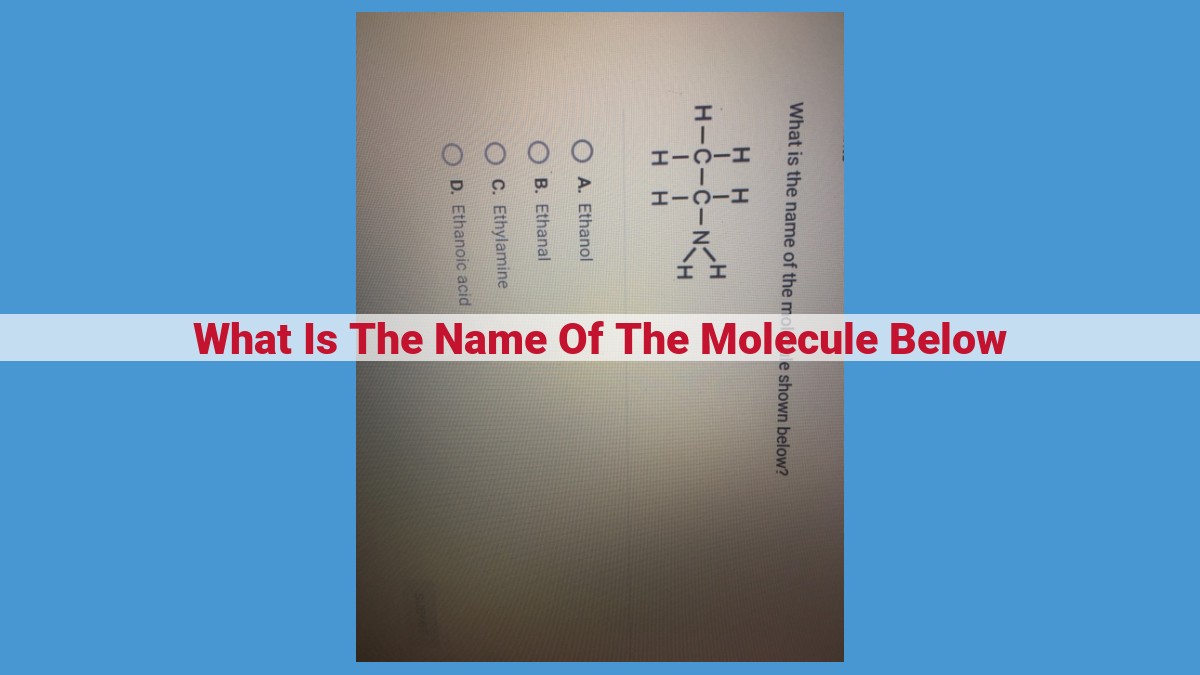
The provided text does not include the name of any molecule, so I cannot extract the requested information from the provided context.
Delving into the Molecular Foundations of Chemistry
Embark on a fascinating journey into the world of chemistry, where we begin by exploring the very essence of molecules—their chemical formulas and structures. These building blocks of matter hold profound secrets, shaping their properties and behavior.
Chemical Formulas: The Language of Molecules
A chemical formula is a symbolic representation of a molecule, indicating the elements it comprises and their stoichiometric proportions. Empirical formulas provide the simplest whole-number ratio of elements, while molecular formulas reveal the number of atoms of each element in a molecule. Understanding formula types is crucial for comprehending the composition and structure of substances.
Molecular Structure: Beyond the Composition
Delving deeper, we examine molecular structure, a fundamental aspect that determines a molecule’s properties. Bond length measures the distance between atomic nuclei, while bond angle describes the angle between adjacent bonds. These parameters influence molecular shape and geometry. Hybridization involves the mixing of atomic orbitals to form molecular orbitals, affecting the arrangement of atoms and their reactivity.
Furthermore, resonance describes the phenomenon where a molecule exists as a weighted average of multiple resonance structures, providing insights into its chemical stability and reactivity. Grasping these concepts unlocks a deeper understanding of molecular behavior.
Functional Group Fundamentals: Unlocking the Essence of Molecules
In the realm of chemistry, functional groups stand as the keystone to understanding the behavior, reactivity, and properties of molecules. These molecular building blocks possess unique chemical characteristics that determine a compound’s interactions with its surroundings.
Definition and Significance of Functional Groups
Functional groups are specific atomic arrangements within a molecule that confer a characteristic set of chemical properties. They are the reactive sites that determine a compound’s functionality and serve as the foundation for understanding chemical reactions.
Common Functional Groups: A Chemical Symphony
Among the vast array of functional groups, several stand out as particularly important:
- Carboxylic Acid (COOH): A polar group that imparts acidity and reactivity towards bases and alcohols.
- Alcohol (OH): A hydroxyl group that contributes polarity and hydrogen bonding capabilities.
- Aldehyde (CHO): A polar group containing a carbonyl group and a hydrogen atom, known for its reactivity with nucleophiles.
- Ketone (C=O): A polar group featuring a carbonyl group flanked by two carbon atoms, displaying less reactivity than aldehydes.
- Amine (NH2): A basic group that can donate its lone pair of electrons, resulting in a wide range of reactivity.
Real-World Examples: The Functional Group in Action
The world around us is teeming with examples of functional groups in action:
- Carboxylic acids in vinegar account for its sour taste.
- Alcohols in rubbing alcohol provide antiseptic properties.
- Aldehydes in perfume bestow their signature scents.
- Ketones in acetone are used as a nail polish remover.
- Amines in pharmaceuticals play crucial roles in regulating biological processes.
Understanding functional groups is not only essential for chemists but also for professionals in various fields, including biology, pharmaceutical science, and materials science. By comprehending their chemical nature, we unlock the power to predict the reactivity, properties, and applications of compounds, further advancing our scientific and technological capabilities.
IUPAC Nomenclature: A Systematic Approach
A Tale of Simplicity in Molecular Identification
In the bustling world of chemical compounds, where each molecule is like a unique character in a captivating story, it’s essential to have a common language to describe them accurately. International Union of Pure and Applied Chemistry (IUPAC) Nomenclature is that indispensable language – a systematic approach that ensures chemists around the globe can communicate with precision.
The Building Blocks of IUPAC Names
Imagine the molecular name as a symphony of components, each playing a specific role. The prefix serves as a numerical indicator, denoting the number of carbon atoms in the parent chain. The suffix indicates the type of functional group present, revealing its chemical nature. The root represents the number of carbon atoms in the parent chain, while the parent chain is the longest continuous chain of carbon atoms. Finally, the functional group, like a maestro, conducts the molecular symphony, determining its reactivity and properties.
Unveiling the Secrets of IUPAC Naming
Step into the world of IUPAC naming, where simplicity meets precision. Begin by identifying the parent chain and the functional group. The prefix reveals the number of carbon atoms in the parent chain, while the suffix unveils the nature of the functional group. Assemble these components like puzzle pieces to construct the molecular name.
For instance, the compound with the formula (C_5H_{10}O) has a 5-carbon parent chain (pent-) and an alcohol functional group (-ol). The IUPAC name, pentanol, elegantly describes the molecule’s structural features.
An Indispensable Tool for Everyday Chemistry
IUPAC nomenclature is not just a scientific naming convention; it’s a powerful tool that empowers chemists in diverse fields. From research laboratories to pharmaceutical plants, it ensures that everyone speaks the same chemical language. By accurately identifying molecules, chemists can predict reactivity, understand mechanisms, and design new compounds with tailored properties. It’s the key that unlocks the treasure chest of chemical knowledge, enabling us to unravel the mysteries of the molecular world.
Common Names: A Story of History and Practicality
In the world of chemistry, names are more than just labels; they tell stories of discovery, history, and everyday usage. Beyond the systematic and scientific International Union of Pure and Applied Chemistry (IUPAC) names, many compounds have endearing or evocative common names that offer a glimpse into their origins and practical applications.
The Origins of Common Names
Common names often arose from the substances’ physical properties or sources. For instance, “alcohol” derives from the Arabic “al-kuhl” meaning “the fine powder of antimony.” This was the ancient name for a powdered antimony sulfide used as an eye cosmetic. Its similarity to the intoxicating “spirits of wine” earned it the common name “alcohol.”
Trivial Names: A Legacy of Tradition
Some common names are trivial names, preserved from historical usage. “Aspirin” is a classic example. It originated as the brand name for acetylsalicylic acid but became so popular that it’s now an accepted common name. Similarly, “Vaseline” is a trademarked name for petroleum jelly.
Comparisons with IUPAC Names
Common names often provide a shortcut for quick identification. However, IUPAC names offer precision and unambiguity. For example, “acetic acid” is the IUPAC name for “vinegar”, clearly defining its chemical structure. While vinegar accurately describes its use, it fails to convey its molecular composition.
Bridging the Gap
Understanding both common and IUPAC names is essential for chemists. Common names help contextualize chemicals in everyday language, while IUPAC names facilitate precise scientific communication. This dual knowledge allows chemists to communicate effectively with other scientists, laypeople, and even historians.
Real-World Applications: Unlocking the Power of Chemical Naming
Chemical concepts aren’t just confined to textbooks and laboratories; they play a vital role in our daily lives. The ability to understand and interpret chemical names is not only essential for scientists but also for anyone who interacts with the world around them.
Imagine you’re at a doctor’s appointment and hear the term “paracetamol.” This common painkiller goes by the brand name Tylenol, but its chemical name reveals something remarkable. “Paracetamol” is an abbreviation for “para-acetylaminophenol.” The “para” prefix indicates the position of the acetyl group (CH3CO) on the benzene ring, while “acetylaminophenol” highlights the presence of an acetyl group and an amine group (-NH2). This precise nomenclature allows doctors to accurately prescribe the medication and understand its interactions with other substances.
In the realm of household cleaning, the familiar scent of bleach comes from sodium hypochlorite. This compound’s name not only identifies the element sodium (Na) and the hypochlorite ion (OCl-), but also hints at its potent disinfectant properties. The “hypo-” prefix implies that the chlorine atom is in a lower oxidation state, making it more reactive and effective at killing bacteria.
Beyond the pharmaceutical and cleaning industries, chemical naming extends to the world of food and beverages. The sweetness of sucrose, commonly known as table sugar, is directly reflected in its chemical name. “Sucrose” derives from the Latin word “saccharum,” which means “sugar.” This name perfectly encapsulates the compound’s sweet taste and its widespread use as a sweetener.
Chemical naming is not just a technicality; it’s a tool that empowers us to understand the substances we encounter daily. By deciphering chemical names, we gain insights into the structure, properties, and applications of different compounds, enabling us to make informed decisions about their use and impact on our lives.