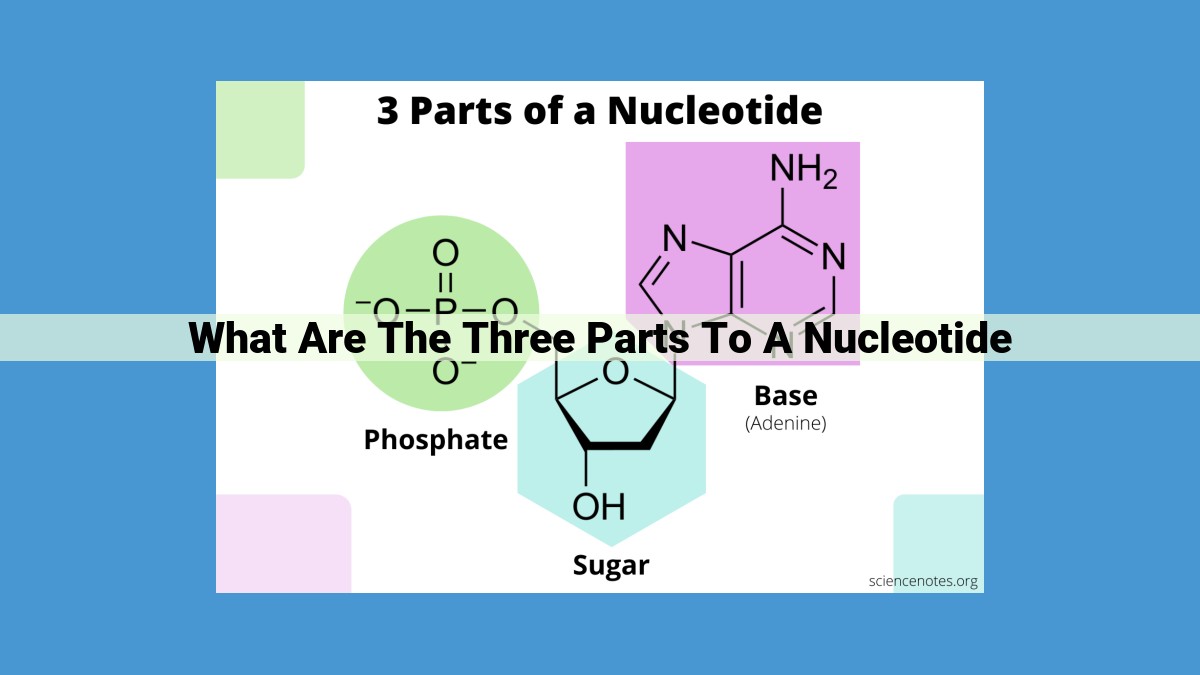
Nucleotides, the building blocks of nucleic acids, consist of three key components: a nitrogenous base (purine or pyrimidine), a deoxyribose or ribose sugar, and a phosphate group. The base-sugar combination forms a nucleoside, which binds to the phosphate group to create the nucleotide. Nucleotides play vital roles in biology, serving as the building blocks of DNA and RNA, energy carriers in metabolism, and coenzymes in metabolic reactions. Their acidic nature and hydrogen bonding capacity contribute to their diverse functions in biological systems.
The Building Blocks of Life: Unveiling the Secrets of Nucleotides
In the intricate tapestry of life, nucleotides form the very essence of our biological processes. They are the essential building blocks of the molecules that make up our DNA, RNA, and provide energy for countless cellular functions. Join us on an adventure as we delve into the fascinating world of nucleotides, exploring their remarkable composition and the vital roles they play in the symphony of life.
Essential Components of a Nucleotide
A nucleotide is not merely a single entity but a complex trio of components that work in harmony:
- Nitrogenous bases: The purines adenine and guanine, and the pyrimidines cytosine, thymine, and uracil. These bases are the key identifiers that determine a nucleotide’s role.
- Deoxyribose or ribose sugar: The sugar backbone that forms the nucleotide’s structural foundation.
- Phosphate group: This high-energy molecule provides the fuel that drives many biological reactions.
Structure and Bonding
These components are linked together through intricate covalent bonds, forming a nucleotide’s unique architecture. The nitrogenous base and sugar form a nucleoside, while the addition of a phosphate group creates a nucleotide. The acidic nature of the phosphate group allows nucleotides to form hydrogen bonds, which are crucial for the pairing of nucleotides in DNA and RNA.
Vital Roles in Biological Systems
Nucleotides are not just passive spectators in the biological drama; they play indispensable roles that sustain life:
- Formation of DNA and RNA: These molecules are the blueprints of our genetic information, carrying the instructions for the development and functioning of all living organisms.
- Energy carriers in metabolism: Nucleotides like ATP (adenosine triphosphate) and GTP (guanosine triphosphate) are the primary energy currencies of cells, providing the power for countless cellular processes.
- Coenzymes in metabolic reactions: Nucleotides such as NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) act as molecular helpers, facilitating and regulating metabolic reactions.
Essential Components of a Nucleotide: The Building Blocks of Life
At the heart of every living organism, there lies a symphony of intricate molecular structures called nucleotides. These tiny molecules, often referred to as the “building blocks” of life, play a pivotal role in an array of biological processes, from the storage and transmission of genetic information to the transfer of energy within cells.
To understand their profound significance, let’s delve into the essential components that make up a nucleotide:
1. Nitrogenous Base:
The nitrogenous base is the foundational unit of a nucleotide. These compounds, which can be either purines (adenine and guanine) or pyrimidines (cytosine, thymine, and uracil), provide the nucleotide with its unique identity and function.
2. Deoxyribose or Ribose Sugar:
The sugar component, either deoxyribose or ribose, forms the backbone of the nucleotide. This sugar molecule, with its intricate arrangement of carbon and oxygen atoms, provides the framework for the nucleotide’s structure and bonding.
3. Phosphate Group:
The phosphate group, a molecule containing phosphorus and oxygen atoms, plays a dual role in a nucleotide. It not only provides the negative charge to the nucleotide but also serves as a key component in energy storage and transfer.
Together, these essential components form the foundation of nucleotides, the molecules that underpin the very fabric of life. Their unique structure and chemical properties enable them to perform an astonishing array of functions within biological systems, shaping the destiny of every living organism.
Structure and Bonding: The Blueprint of Nucleotides
In unraveling the secrets of life’s intricate tapestry, nucleotides play a pivotal role. Their structure, like the blueprint of a grand edifice, dictates their diverse functions in biological systems.
Nucleobase-Nucleoside and Nucleoside-Phosphate Bonds: The Cornerstones of Nucleotide Architecture
Nucleotide assembly begins with nucleobases, the nitrogen-rich building blocks that differentiate the nucleotides. These bases, classified as purines (adenine and guanine) and pyrimidines (cytosine, thymine, and uracil), form a bond with a nucleoside, a five-carbon sugar molecule ( ribose or deoxyribose).
The final touch is the addition of a phosphate group, which forms a covalent bond with the nucleoside, giving the nucleotide its characteristic negative charge. This trio—nucleobase, nucleoside, and phosphate—forms the fundamental core of a nucleotide.
Acidic Nature and Hydrogen Bonding Capacity: The Keys to Nucleotide Interactions
The phosphate group imparts an acidic nature to nucleotides, allowing them to readily donate hydrogen ions and form ionic bonds. Additionally, hydrogen bonding plays a crucial role in nucleotide interactions. The electronegative nitrogen and oxygen atoms on the nucleobases can both accept and donate hydrogen bonds, leading to the formation of highly specific and stable structures.
These hydrogen bonds are the driving force behind the famous base pairing in DNA and RNA. Adenine pairs with thymine (or uracil in RNA), while guanine pairs with cytosine, forming the backbone of these essential information-carrying molecules.
The Multifaceted Roles of Nucleotides in Life’s Processes
Guardians of Genetic Information
Nucleotide building blocks, the foundation of DNA and RNA, safeguard the blueprints of life. These intricate molecules store and transmit genetic information, ensuring the continuity of species across generations. DNA, with its double helix structure, harbors the complete genetic code, while RNA serves as a messenger, carrying instructions from DNA to ribosomes for protein synthesis. Without nucleotides, the very essence of life would crumble.
Powerhouses of Metabolism
Beyond their genetic prowess, nucleotides play a pivotal role in the energy currency of cells. ATP (adenosine triphosphate) and GTP (guanosine triphosphate), high-energy molecules, fuel countless cellular processes. Through the hydrolysis of their phosphate bonds, they release energy to power everything from muscle contractions to nerve impulses, keeping the body’s machinery running smoothly.
Co-Stars in Metabolic Reactions
Nucleotide derivatives, such as NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide), serve as coenzymes, essential partners in metabolic reactions. They act as electron carriers, enabling the transfer of energy and facilitating a myriad of biochemical transformations. Without these coenzymes, metabolism would grind to a halt, depriving cells of vital nutrients and energy.
Nucleotide components, including the nitrogenous base, sugar, and phosphate group, assemble to form the building blocks of DNA and RNA, the carriers of genetic information. They provide energy for cellular processes through ATP and GTP and participate in metabolic reactions as coenzymes. The diversity of nucleotide roles underscores their importance as the foundation of life itself.