To determine the percent ionic character, calculate the difference in electronegativity between bonded atoms using the Pauling Electronegativity Scale. Employ the formula % Ionic Character = 100(1 – e^(-0.25∆EN^2)), where ∆EN represents the electronegativity difference. This formula quantifies the extent of electrostatic attraction between charged atoms, indicating the bond’s polarity and ionic character.
Definition of Percent Ionic Character
- Explain that percent ionic character describes the extent of electrostatic attraction between charged atoms in a bond.
How to Get a Grip on Percent Ionic Character: A Beginner’s Guide
Imagine you’re trying to figure out how strong your friendship is. You could ask about the time you stayed up all night talking, or the number of secrets you’ve shared. Well, chemists do something similar to understand the strength of chemical bonds. And one way they do this is by looking at percent ionic character.
What’s Percent Ionic Character All About?
Percent ionic character is like a measure of how much a bond between two atoms acts like a magnet. When atoms bond, they can share electrons or steal them from each other, creating charged atoms called ions. The more ionic a bond is, the stronger the attraction between these ions.
Key Ingredients
To understand percent ionic character, let’s dive into some key concepts:
- Electronegativity: This is how much an atom likes to hog electrons. The higher the electronegativity, the more it wants to pull electrons towards itself.
- Difference in Electronegativity: When two atoms bond, the difference in their electronegativity determines how ionic the bond will be. The bigger the difference, the more ionic the bond.
- Pauling Electronegativity Scale: This scale gives each element a number that represents its electronegativity. It’s like a cheat sheet for predicting how ionic a bond will be.
The Formula: A Map to Ionic Character
Now, let’s get to the nitty-gritty: the formula for percent ionic character. It’s like a recipe that tells us how much ionic character is in a bond:
% Ionic Character = 100 x (1 - e^(-(ΔEN)²/4))
where:
- ΔEN is the difference in electronegativity
- e is the base of the natural logarithm (approx. 2.718)
That exponential term (the part with the e) is like a magnifying glass. It amplifies small differences in electronegativity, making them have a big impact on ionic character.
Key Concepts
- Electronegativity: Define electronegativity and explain its role in determining bond polarity.
- Difference in Electronegativity: Discuss how the difference in electronegativity between bonded atoms affects the bond’s ionic character.
- Pauling Electronegativity Scale: Introduce the scale and its use in quantifying electronegativity.
Key Concepts in Determining Percent Ionic Character
Understanding the extent of electrostatic attraction in chemical bonds is crucial for comprehending their properties. Percent ionic character quantifies this attraction, providing valuable insights into the nature of bonds. To determine percent ionic character, it’s essential to grasp a few key concepts.
Electronegativity: The Affinity for Electrons
Electronegativity is the ability of an atom to attract electrons within a chemical bond. It arises from the interplay between the atom’s nuclear charge and its electron configuration. Elements with high electronegativity, such as fluorine, have a strong pull on electrons, while those with low electronegativity, like sodium, have a weaker grip.
In a bond between two different elements, the more electronegative atom draws electrons towards itself, creating an uneven distribution of charge. This imbalance leads to a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom.
Difference in Electronegativity: The Driving Force of Ionic Character
The difference in electronegativity between bonded atoms is a significant factor in determining the bond’s ionic character. The greater the difference in electronegativity, the more polarized the bond will be, and the higher its ionic character.
For example, a bond between sodium (electronegativity = 0.93) and chlorine (electronegativity = 3.0) has a large difference in electronegativity of 2.07. This significant difference results in a highly polarized bond with a substantial ionic character.
Pauling Electronegativity Scale: Quantifying Electronegativity
To quantify electronegativity, chemists use the Pauling Electronegativity Scale. As a numerical scale, it assigns values to different elements based on their ability to attract electrons. Higher values indicate greater electronegativity.
The scale is named after Linus Pauling, who developed it in the 1930s. It provides a convenient and standardized method for comparing the electronegativities of different elements and understanding their potential to form ionic bonds.
Formula for Percent Ionic Character
To quantify the ionic character of a bond, we use the following formula:
% Ionic Character = 100 * (1 - e^(-0.25 * (ΔEN)^2))
Here, the components of the equation are:
-
ΔEN: The difference in electronegativity between the bonded atoms, which is calculated by subtracting the electronegativity of the less electronegative atom from the electronegativity of the more electronegative atom.
-
0.25: A constant used to scale the electronegativity difference to a more appropriate range for percent ionic character calculations.
-
e: The base of the exponential function (approximately 2.718).
The significance of the exponential term is that it dampens the effect of the electronegativity difference. This means that even large differences in electronegativity do not necessarily result in an extremely high percent ionic character. The exponential function ensures that the percent ionic character increases gradually as the electronegativity difference increases.
Steps to Calculate Percent Ionic Character
To determine the extent of electrostatic attraction in a bond, we calculate its percent ionic character. Here’s a step-by-step guide:
1. Identifying the Bonded Atoms
First, identify the two atoms forming the bond. The difference in their electronegativity, a measure of an atom’s ability to attract electrons, plays a crucial role.
2. Finding Electronegativity Values
Next, look up the electronegativity values of the bonded atoms using the Pauling Electronegativity Scale. Higher electronegativity indicates a greater tendency to pull electrons.
3. Calculating the Difference in Electronegativity
Subtract the electronegativity of the less electronegative atom from that of the more electronegative atom. The resulting value represents their difference in electronegativity.
4. Substituting into the Equation
Use the following formula to calculate the percent ionic character:
Percent Ionic Character = 100% * (1 - e^(-ΔEN/4))
where:
- ΔEN is the difference in electronegativity
5. Evaluating the Result
The result will be a percentage ranging from 0% to 100%. Higher values indicate a more ionic character, while lower values suggest a more covalent character.
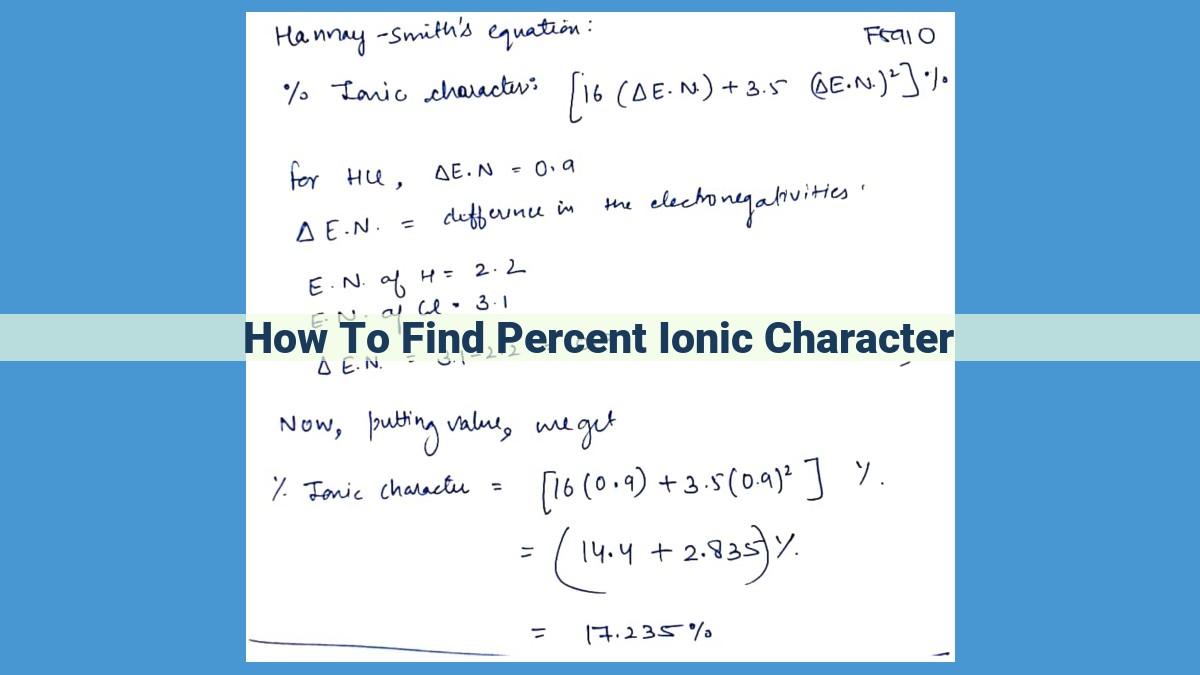
Example Calculation
Consider the sodium-fluorine bond, where sodium has an electronegativity of 0.9 and fluorine has an electronegativity of 4.0.
-
Difference in electronegativity (ΔEN) = 4.0 – 0.9 = 3.1
-
Plugging into the formula:
Percent Ionic Character = 100% * (1 - e^(-3.1/4)) = 97.7%
This result indicates that the sodium-fluorine bond has a high ionic character, meaning that the electrostatic attraction between charged ions dominates the bond’s interactions.
Determining Percent Ionic Character: A Comprehensive Guide
We delve into the fascinating world of chemical bonding as we explore the concept of ionic character. Percent ionic character quantifies the electrostatic attraction between charged atoms in a chemical bond. This article will provide a comprehensive guide to understanding percent ionic character, its key concepts, formula, calculation steps, and implications.
Key Concepts
- Electronegativity: Electronegativity measures an atom’s ability to attract electrons towards itself in a chemical bond. The Pauling Electronegativity Scale assigns values to elements, with higher values indicating greater electronegativity.
- Difference in Electronegativity: The difference in electronegativity between bonded atoms influences the bond’s ionic character. A larger difference indicates a more polar bond and a greater ionic character.
Calculating Percent Ionic Character
The formula for percent ionic character is:
% Ionic Character = 100% * (1 - e^(-0.25 * ΔEN^2))
where:
- ΔEN is the absolute difference in electronegativity between the bonded atoms.
Example Calculation
Consider the bond between sodium (Na) and chlorine (Cl). Their electronegativity values are approximately 0.9 and 3.0, respectively.
- ΔEN = |3.0 – 0.9| = 2.1
- % Ionic Character = 100% * (1 – e^(-0.25 * 2.1^2))
- % Ionic Character ≈ 92.8%
Implications of Percent Ionic Character
Percent ionic character has significant implications for bond properties:
- Bond Strength: Ionic bonds are typically stronger than covalent bonds. Higher ionic character indicates stronger bonds.
- Bond Polarity: Ionic bonds are polar, with electrons concentrated around the more electronegative atom. Higher ionic character corresponds to greater bond polarity.
Limitations of Percent Ionic Character
While percent ionic character provides a useful measure of bond character, it has certain limitations:
- Oversimplification: Percent ionic character treats bonds as either completely ionic or covalent, which may not accurately reflect reality.
- Charge Separation: The exponential term in the formula emphasizes charge separation, which may not fully capture other factors influencing ionic character.
Understanding percent ionic character is essential for comprehending the nature of chemical bonds. By determining the extent of electrostatic attraction between bonded atoms, we can gain insights into bond strength, polarity, and other important properties. While the formula for percent ionic character provides a valuable tool, it is important to consider its limitations when interpreting bond characteristics.
The Significance of Percent Ionic Character: Unraveling its Impact on Bond Properties
Bond Strength: A Tale of Electrostatic Attraction
The percent ionic character of a bond significantly influences its bond strength. As the ionic character increases, so does the electrostatic attraction between the bonded atoms. This enhanced attraction leads to stronger bonds. Conversely, bonds with low ionic character exhibit weaker bonds due to reduced electrostatic attraction.
Polarity Puzzle: Unveiling the Nature of Bonds
Bond polarity is heavily influenced by the percent ionic character. In bonds with high ionic character, the electron density is unevenly distributed, resulting in a polar bond. This polarity arises from the electrostatic attraction between the oppositely charged ions. In contrast, bonds with low ionic character are more nonpolar, as the electrons are equally shared between the bonded atoms.
Delving into Applications: Ionic Character’s Practical Relevance
Understanding the percent ionic character of bonds is crucial in various scientific fields. In materials science, it helps predict material properties such as strength, conductivity, and thermal stability. In organic chemistry, it aids in comprehending chemical reactivity and molecular behavior. Furthermore, in biochemistry, it provides insights into the structure and function of biological molecules.
Remember: The percent ionic character of a bond is a measure of its electrostatic character. It impacts bond strength and polarity, shedding light on a bond’s nature and behavior. Understanding this concept is essential in chemistry and its practical applications.
Limitations of Percent Ionic Character
Percent ionic character provides valuable insights into the nature of chemical bonds, but it’s essential to recognize its limitations:
-
Oversimplification of Bond Character: Percent ionic character assumes a clear distinction between ionic and covalent bonds. However, in reality, bonds exist on a continuum between these extremes, and quantifying the ionic character may not fully capture the complex nature of bond interactions.
-
Neglecting Resonance and Delocalization: Percent ionic character doesn’t account for resonance or delocalization, where electrons are spread over multiple atoms. In such cases, the ionic character may be overestimated, as the electrostatic attraction between charged atoms is not solely responsible for the bond strength.
-
Assumptions about Electronegativity: Percent ionic character assumes that electronegativity accurately reflects the tendency of atoms to attract electrons. While electronegativity provides a useful approximation, it doesn’t always perfectly represent the charge distribution in a bond. Factors like atomic size and hybridization can affect the electron distribution, leading to discrepancies in the calculated ionic character.
-
Dependence on a Single Parameter: Percent ionic character relies solely on the difference in electronegativity. However, other factors such as bond length, bond order, and hybridization also influence bond properties. By considering only electronegativity, percent ionic character can overlook these other important factors.
-
Inaccuracy for Highly Polarized Bonds: Percent ionic character is most useful for bonds with significant ionic character. For highly polarized or near-ionic bonds, the calculated ionic character may approach 100%, which may not accurately reflect the bond’s true nature.
Understanding these limitations is crucial for interpreting the percent ionic character and avoiding misinterpretations in assessing bond character.