Percent dissociation measures the fraction of a compound that dissociates into ions in solution. To calculate percent dissociation, determine the equilibrium concentration of the dissociated ions and divide by the initial concentration of the compound. Use an ICE table to establish equilibrium concentrations. Employ the equilibrium expression and percentage expression to calculate the percentage of the compound that dissociates. This concept applies to weak acids and bases, where understanding percent dissociation provides insights into acid-base strength, equilibrium dynamics, and solubility.
Understanding Percent Dissociation: The Key to Demystifying Equilibria
In the realm of chemistry, understanding percent dissociation is akin to unlocking a secret code that deciphers the behavior of substances in solution. Percent dissociation measures the extent to which a solute breaks down into ions in water. This knowledge empowers us to grasp the strength of acids and bases, the intricacies of acid-base equilibria, and the solubility of compounds.
Let’s envision a weak acid like acetic acid, a common ingredient in vinegar. When acetic acid dissolves in water, it undergoes a reversible reaction, dissociating into hydrogen ions (H+) and acetate ions (CH3COO-):
CH3COOH (aq) + H2O (l) ⇌ H+ (aq) + CH3COO- (aq)
The extent to which this reaction proceeds is crucial, as it dictates the concentration of H+ ions in solution, which in turn determines the acidity of the solution. Percent dissociation provides a quantitative measure of this extent.
It is defined as the percentage of the initial concentration of the weak acid that has dissociated into ions:
Percent dissociation = (Concentration of dissociated solute / Initial concentration of solute) x 100%
This value helps us understand the relative strength of acids and bases: Strong acids dissociate nearly completely, resulting in a high percent dissociation, while weak acids dissociate only partially, yielding a lower percent dissociation.
Comprehension of percent dissociation forms the cornerstone for exploring the behavior of solutions, unlocking the secrets of chemical equilibria, and unraveling the mysteries of intermolecular interactions.
Related Concepts in Understanding Percent Dissociation
In the realm of chemistry, the concept of percent dissociation is central to understanding the behavior of weak acids and bases. To fully grasp percent dissociation, it’s essential to familiarize ourselves with three key related concepts: initial concentration, equilibrium concentration, and equilibrium constant.
Initial Concentration:
The initial concentration refers to the concentration of the weak acid or base before it undergoes dissociation. This value represents the starting point for the reaction and is typically expressed in molarity (M).
Equilibrium Concentration:
As the reaction proceeds, the weak acid or base partially dissociates into its constituent ions. The equilibrium concentration represents the concentrations of the reactants and products at equilibrium, where the forward and reverse reactions occur at equal rates.
Equilibrium Constant:
The equilibrium constant, denoted by K, is a constant value that characterizes the extent to which a reaction will proceed to completion. It is the ratio of the equilibrium concentrations of the products to the equilibrium concentrations of the reactants. The larger the equilibrium constant, the more dissociation occurs.
These three concepts are intertwined and form the foundation for understanding percent dissociation. By understanding the relationships between initial concentration, equilibrium concentration, and equilibrium constant, we can gain valuable insights into the behavior of weak acids and bases.
Calculating Percent Dissociation
- Step-by-step formula and explanation.
Calculating Percent Dissociation
Percent dissociation is a crucial concept in understanding the behavior of weak acids and bases in solution. It measures the extent to which a solute dissociates into its ions, providing valuable insights into the strength of the acid or base and its equilibrium properties.
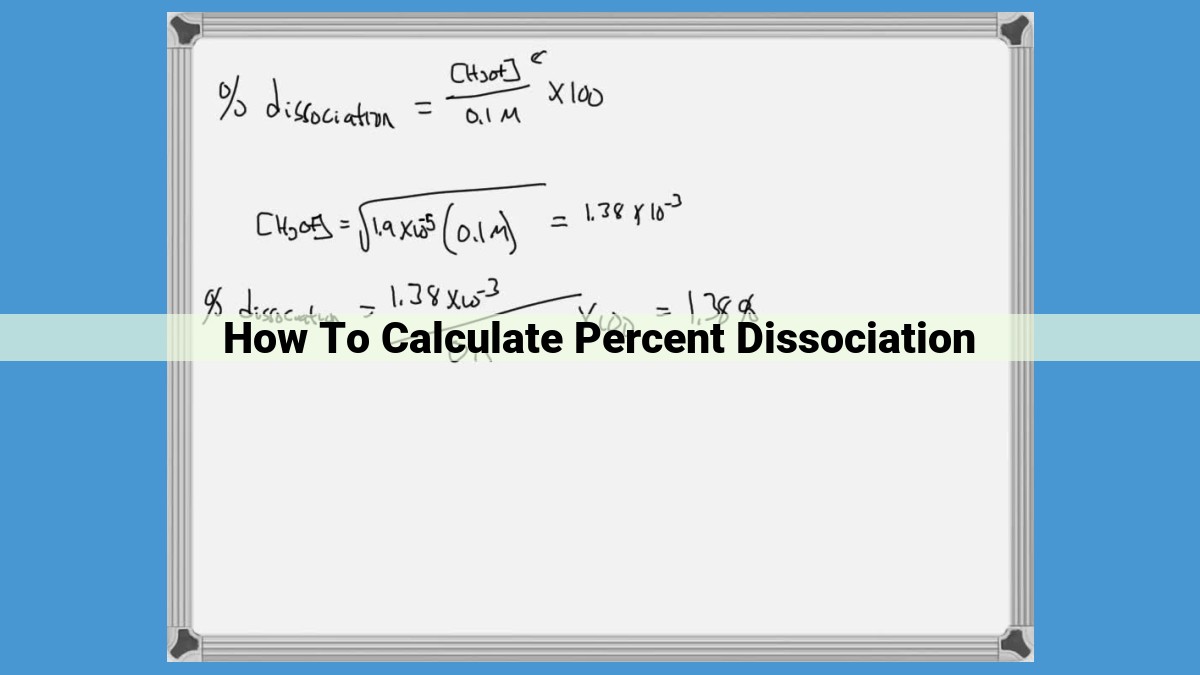
To calculate percent dissociation (α), we utilize the following formula:
α = (Equilibrium concentration of ions) / (Initial concentration of solute) x 100%
This formula highlights the relationship between the initial concentration of the solute, which represents the total amount of acid or base present, and the equilibrium concentration of ions, which indicates the amount that has dissociated.
Step-by-Step Calculation:
- Determine the equilibrium concentration of ions: This can be achieved using an ICE table (Initial, Change, Equilibrium) or by using the equilibrium constant (K_eq) and the initial concentration of the solute.
- Substitute the values into the formula: Once you have the equilibrium concentration of ions, plug it into the formula along with the initial concentration of the solute.
- Calculate percent dissociation: Multiply the result by 100% to obtain the percentage value.
By understanding and applying this formula, you can determine the percent dissociation of weak acids and bases, which is essential for comprehending acid-base equilibria and various chemical reactions in solution.
**Unveiling the Secrets of Equilibrium Concentrations: Using an ICE Table**
In the realm of chemistry, understanding the behavior of chemical reactions at equilibrium is crucial. One essential tool for deciphering this intricate dance is the ICE table. It’s like having a secret weapon, empowering us to determine the equilibrium concentrations of reactants and products with remarkable accuracy.
An ICE table, short for Initial Concentration, Change, Equilibrium Concentration, is a strategic grid that tracks the evolution of chemical species during a reaction. We start by filling in the initial concentrations of each reactant and product based on the given conditions.
As the reaction proceeds, changes occur. Some reactants are consumed, while products are formed. The change column quantifies these transformations, typically represented by x. This x value signifies the amount of species that react or form.
Finally, the equilibrium concentrations are calculated by combining the initial concentrations and the changes. These are the concentrations at which the reaction has reached a standstill, with no further net change.
Using an ICE table is a systematic and straightforward process. Let’s delve into its steps:
- Set up the table: Construct a grid with rows for each species and columns for initial concentration, change, and equilibrium concentration.
- Fill in the initial concentrations: Enter the provided initial concentrations in the appropriate rows.
- Write the chemical equation: Balance the chemical equation and place it above the table.
- Determine the stoichiometry: Use the balanced equation to establish the stoichiometric ratios between reactants and products.
- Calculate the change: In the change column, enter the stoichiometric coefficients multiplied by x. For reactants, this value is negative, indicating their consumption. For products, it’s positive, representing their formation.
- Calculate the equilibrium concentrations: Sum the initial concentration and the change to obtain the equilibrium concentration.
Mastering the ICE table is like unlocking a treasure trove of chemical information. It empowers us to predict the equilibrium behavior of reactions, allowing us to unravel the mysteries of chemical equilibria and gain a deeper understanding of the molecular world around us.
Equilibrium Expression: A Deeper Dive
In the realm of weak acids, the concept of percent dissociation plays a pivotal role in comprehending their behavior and strength. This enigmatic expression, which serves as a quantitative measure of the extent to which a weak acid ionizes in solution, has profound implications for various chemical phenomena.
The equilibrium expression is a mathematical equation that precisely captures the relationship between the concentrations of the various species present in an acid dissociation equilibrium. For a weak acid, such as HA, this expression takes the form:
**Ka = [H+][A-] / [HA]**
where:
- Ka is the equilibrium constant for the acid dissociation reaction
- [H+] is the molar concentration of hydronium ions (H+)
- [A-] is the molar concentration of the conjugate base (A-)
- [HA] is the molar concentration of the undissociated acid (HA)
This expression represents the law of mass action, which states that the equilibrium constant for a chemical reaction is equal to the ratio of the product concentrations to the reactant concentrations, each raised to their respective stoichiometric coefficients. In the case of weak acid dissociation, the equilibrium constant is a constant value that reflects the inherent tendency of the acid to release H+ ions.
By carefully examining the equilibrium expression, we can deduce important insights into the dissociation behavior of weak acids. The larger the value of Ka, the greater the extent of dissociation, indicating a stronger acid. Conversely, a smaller Ka value corresponds to a weaker acid that dissociates less readily. Furthermore, the expression reveals that the equilibrium concentrations of the species involved are inversely proportional to Ka. This means that as the acid becomes stronger (higher Ka), the concentration of undissociated acid decreases, while the concentrations of hydronium ions and conjugate base increase accordingly.
Percentage Expression: Unraveling the Extent of Dissociation
As we explore the intricate world of chemical reactions, understanding the extent to which weak acids or bases dissociate in solution becomes paramount. The percentage expression provides a convenient tool for quantifying this dissociation, giving us insights into the strength of these species.
The percentage expression is a mathematical formula that calculates the percent dissociation of a weak acid or base. It expresses the ratio of the number of molecules that have dissociated to the total number of molecules initially present. Using this formula, we can determine the fraction of the initial concentration that has actually undergone dissociation.
To calculate the percent dissociation using the percentage expression, we use the following formula:
Percent Dissociation = ([Dissociated Concentration] / [Initial Concentration]) x 100%
Where:
- [Dissociated Concentration] is the equilibrium concentration of the dissociated species (products of dissociation).
- [Initial Concentration] is the initial concentration of the weak acid or base before dissociation.
By plugging in the equilibrium concentrations, we can obtain a numerical value that represents the percentage of the initial concentration that has dissociated. A higher percentage indicates a greater extent of dissociation, while a lower percentage suggests less dissociation.
Optimizing SEO on Page
- Keywords: Percentage Expression, Percent Dissociation, Weak Acids, Weak Bases, Equilibrium Concentrations
- Image Alt Text: A graph illustrating the relationship between initial concentration and percent dissociation.
- Meta Description: Understanding percent dissociation and how to calculate it using the percentage expression.
- Header Tags:
- H2: Percentage Expression: Unraveling the Extent of Dissociation
- H3: Formula and Calculation
- H3: Applications and Significance
Calculating Percent Dissociation of Weak Acids or Bases: A Step-by-Step Guide
Understanding the concept of percent dissociation is crucial for grasping the behavior of weak acids and bases in solution. To calculate percent dissociation accurately, follow these detailed instructions:
Step 1: Determine the Initial Concentration
Measure or calculate the initial concentration of the weak acid or base, typically denoted as [C_i]. This represents the concentration of the undissociated species at the beginning of the reaction.
Step 2: Establish Equilibrium Concentrations
Set up an ICE table to track the changes in concentrations of the species as the reaction proceeds. Fill in the initial, change, and equilibrium concentrations for the undissociated species, [HA]_i, [HA]_eq, and [A-]_eq, respectively.
Step 3: Calculate Equilibrium Dissociation Constant
Determine the **equilibrium dissociation constant_, K_a, for the weak acid or K_b, for the weak base. K_a and K_b values are inherent properties of the acid or base and can be found in reference tables.
Step 4: Solve for Equilibrium Concentration
Using the ICE table and the equilibrium expression, solve for the equilibrium concentration of the dissociated species, [A-]_eq. The equilibrium expression for a weak acid is:
K_a = [H+][A-] / [HA]
Step 5: Calculate Percent Dissociation
Finally, calculate the percent dissociation, denoted as %D, using the following formula:
%D = ([A-]_eq_ / [C_i_]) x 100%
This formula provides a percentage representation of the extent to which the weak acid or base has dissociated into its constituent ions. A higher percent dissociation indicates a stronger acid or base.
Applications of Percent Dissociation
When discussing the extent to which a solute dissociates into ions in solution, percent dissociation becomes a crucial concept. This measure sheds light on the behavior of various solutes, helping us to understand the strength of acids and bases, acid-base equilibria, and solubility.
Understanding the Strength of Acids and Bases
The percent dissociation of an acid or base provides insights into its strength. A strong acid, such as hydrochloric acid (HCl), dissociates almost completely in water, resulting in a high percent dissociation. Conversely, a weak acid, like acetic acid (CH3COOH), dissociates only partially, leading to a lower percent dissociation. This difference in dissociation behavior reflects the relative strength of the acids.
Acid-Base Equilibria
Percent dissociation also plays a vital role in understanding acid-base equilibria. In a solution containing a weak acid, the extent of dissociation determines the concentration of hydrogen ions (H+)* and the **pH of the solution. A higher percent dissociation indicates a greater concentration of H+ ions and a lower pH, implying a more acidic solution.
Solubility
Furthermore, percent dissociation has implications for the solubility of ionic compounds. Ions in solution can compete with each other for solvation, affecting the solubility of different compounds. A compound with a high percent dissociation will have more ions in solution, potentially reducing the solubility of other ionic compounds.
By comprehending the concept of percent dissociation, we gain valuable insights into the behavior of solutes in solution. This knowledge empowers us to understand the strength of acids and bases, acid-base equilibria, and solubility, equipping us with a deeper understanding of chemical processes.