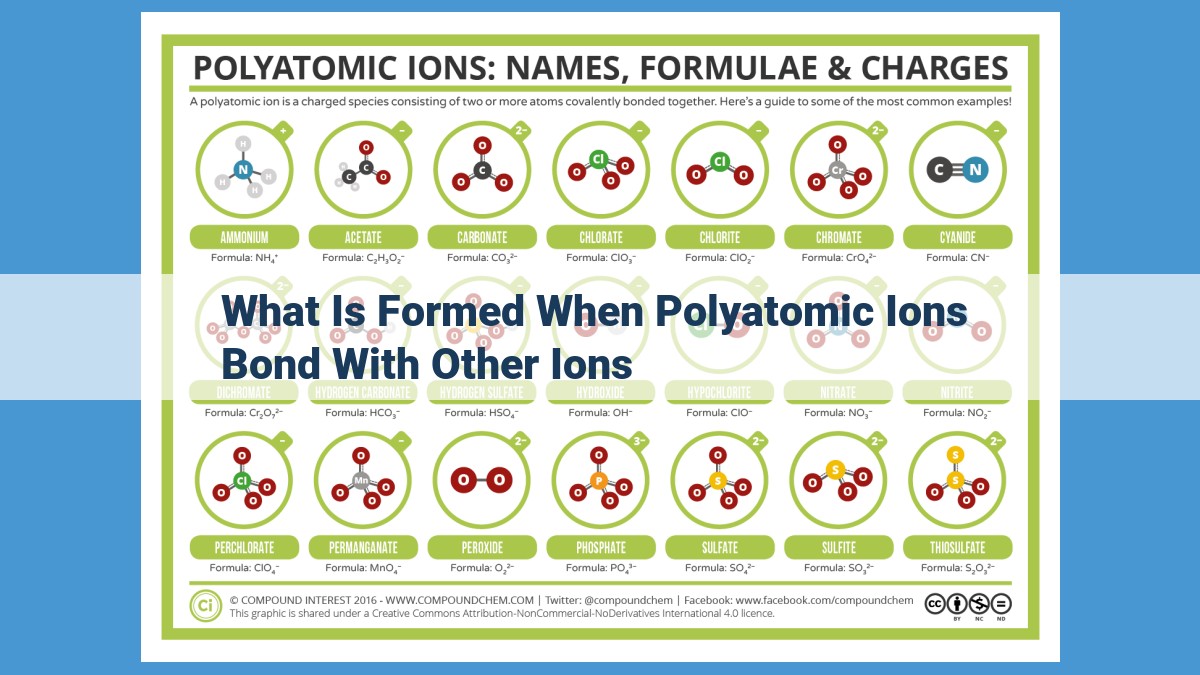
When polyatomic ions, charged groups of atoms, bond with other ions, they form ionic or molecular compounds. Ionic compounds result from electrostatic attraction between polyatomic ions and positive ions, such as sodium and potassium. Molecular compounds involve covalent bonding between polyatomic ions and atoms within a molecule. Polyatomic ions play a crucial role in forming acids, bases, and salts, contributing to their properties and applications in various fields, including chemistry, biology, and environmental science.
Understanding Polyatomic Ions: The Significance in Chemical Interactions
In the captivating world of chemistry, understanding polyatomic ions is crucial for unraveling the mysteries of chemical interactions. Polyatomic ions are fascinating entities, which are charged groups of atoms that form the building blocks of numerous compounds. They play a significant role in various chemical processes, like the formation of acids, bases, and salts.
Picture this: Polyatomic ions are like tiny atomic teams that join forces to create unique identities. These teams consist of multiple atoms of different elements, and they carry an overall electric charge. The charge can be either positive or negative, depending on the arrangement of their electrons.
The significance of polyatomic ions lies in their ability to participate in chemical reactions, paving the way for the formation of complex substances. These ions are like the social butterflies of the chemical world, interacting with other ions and atoms to create a diverse array of compounds. Understanding their behavior is essential for comprehending the intricate tapestry of chemical reactions.
Ionic Bonding with Polyatomic Ions
- Define ionic bonding as the attraction between oppositely charged ions.
- Describe how polyatomic ions bond with positive ions.
- Provide examples of ionic compounds formed by polyatomic ions.
Ionic Bonding with Polyatomic Ions: A Dance of Opposites
Ionic bonding is a captivating dance between oppositely charged ions, where the positive and negative charges create an irresistible attraction, much like a magnet’s pull. This bond forms the foundation of ionic compounds, substances that are composed of these charged particles.
When polyatomic ions, groups of atoms that carry a net charge, enter the ionic bonding dance, they bring a unique twist. These ions, like the nitrate (NO3-) or sulfate (SO42-) ions, have a negative charge that draws them towards positively charged ions, called cations.
As if performing a graceful waltz, the polyatomic ions and cations twirl and align, forming ionic crystals, solid structures where the ions are arranged in a repeating pattern. The strength of this bond depends on the charges of the ions involved, as well as the distance between them. The larger the charge and the smaller the distance, the stronger the bond.
Take, for example, sodium nitrate (NaNO3), an ionic compound formed by sodium ions (Na+), which carry a positive charge, and nitrate ions (NO3-), which carry a negative charge. In this compound, the ions are locked together in a rigid crystal lattice, held captive by the electrostatic attraction between them.
Ionic compounds with polyatomic ions are not just fascinating to study; they also play a crucial role in our everyday lives. The nitrate ion, for instance, is essential for plant growth as it provides nitrogen, a vital nutrient. Sulfate ions, on the other hand, can form salts that are used in everything from fertilizers to detergents.
Understanding the dance of ionic bonding with polyatomic ions not only opens up a world of scientific knowledge but also reveals the hidden chemistry behind many of the substances we encounter daily.
**Molecular Compounds with Polyatomic Ions: A Covalent Connection**
Introduction
Polyatomic ions, those charged groups of atoms, play a crucial role not only in ionic bonding but also in the formation of molecular compounds. In this realm, polyatomic ions dance elegantly with atoms, forming bonds that defy the simple attraction of opposite charges. Let’s unravel the enchanting world of molecular compounds featuring polyatomic ions.
Polyatomic Ions in the Molecular Arena
Molecular compounds, unlike their ionic counterparts, are composed of atoms held together by the strong force of covalent bonds. These bonds arise when atoms share electrons, creating a molecular embrace. Polyatomic ions, with their intricate structures, can participate in this molecular symphony, bonding covalently with atoms to form diverse and fascinating compounds.
Covalent Bonding with Polyatomic Ions
The covalent bond between a polyatomic ion and an atom is a dance of electrons. The polyatomic ion, with its electron-rich nature, can donate electrons to the atom, forming a shared pair that binds them together. This sharing creates a stable molecular entity where the atoms reside in a harmonious equilibrium.
Examples of Molecular Compounds with Polyatomic Ions
The world of molecular compounds containing polyatomic ions is vast and varied. Consider carbon dioxide (CO2), a ubiquitous gas essential for life on Earth. In this molecule, the carbon atom bonds covalently with two oxygen atoms, each harboring a negative charge. Another example is water (H2O), the elixir of life. Here, the hydrogen atoms form covalent bonds with the oxygen atom, creating a polyatomic ion known as hydroxide (OH-).
Conclusion
Polyatomic ions, with their ability to form covalent bonds, expand the horizons of molecular compounds, creating substances with unique properties and applications. Their significance extends far beyond ionic interactions, reaching into the realm of molecular chemistry, where they orchestrate the formation of essential compounds that shape our world. Understanding the intricate dance between polyatomic ions and atoms empowers us to unravel the molecular tapestry of life and the universe at large.
Polyatomic Ions in Acids, Bases, and Salts
In the realm of chemistry, polyatomic ions play a crucial role in the formation of substances that shape our world. These charged groups of atoms not only participate in ionic bonding but also extend their influence to molecular compounds. Their involvement in acids, bases, and salts further highlights their significance in various chemical processes.
Acids
Acids are substances that release hydrogen ions (H+) when dissolved in water. Polyatomic ions contribute to the acidity of solutions by forming anions – negatively charged ions. For instance, the sulfate ion (SO42-) in sulfuric acid (H2SO4) attracts hydrogen ions to form the hydronium ion (H3O+), which is responsible for the acidic properties of the solution.
Bases
Bases, on the other hand, release hydroxide ions (OH-) upon dissolution in water. Polyatomic ions can also contribute to basicity by forming cations – positively charged ions. The ammonium ion (NH4+) in ammonium hydroxide (NH4OH) attracts hydroxide ions, resulting in the formation of the ammonia molecule (NH3) and water.
Salts
Salts are ionic compounds formed by the neutralization of acids and bases. Polyatomic ions can participate in salt formation by interacting with both cations and anions. For example, sodium carbonate (Na2CO3) is formed when sodium ions (Na+) bond with the carbonate ion (CO32-). This salt plays a vital role in various applications, such as glassmaking and the production of detergents.
Examples
- Sulfuric acid (H2SO4): A strong acid containing the sulfate ion (SO42-).
- Ammonium hydroxide (NH4OH): A weak base containing the ammonium ion (NH4+).
- Sodium carbonate (Na2CO3): A salt containing the carbonate ion (CO32-).
Understanding the role of polyatomic ions in acids, bases, and salts is essential for comprehending chemical reactions and their implications. These substances are prevalent in everyday life, from household cleaners to industrial processes. By unraveling the complexities of their formation and interactions, we gain insights into the chemical foundations of our world.