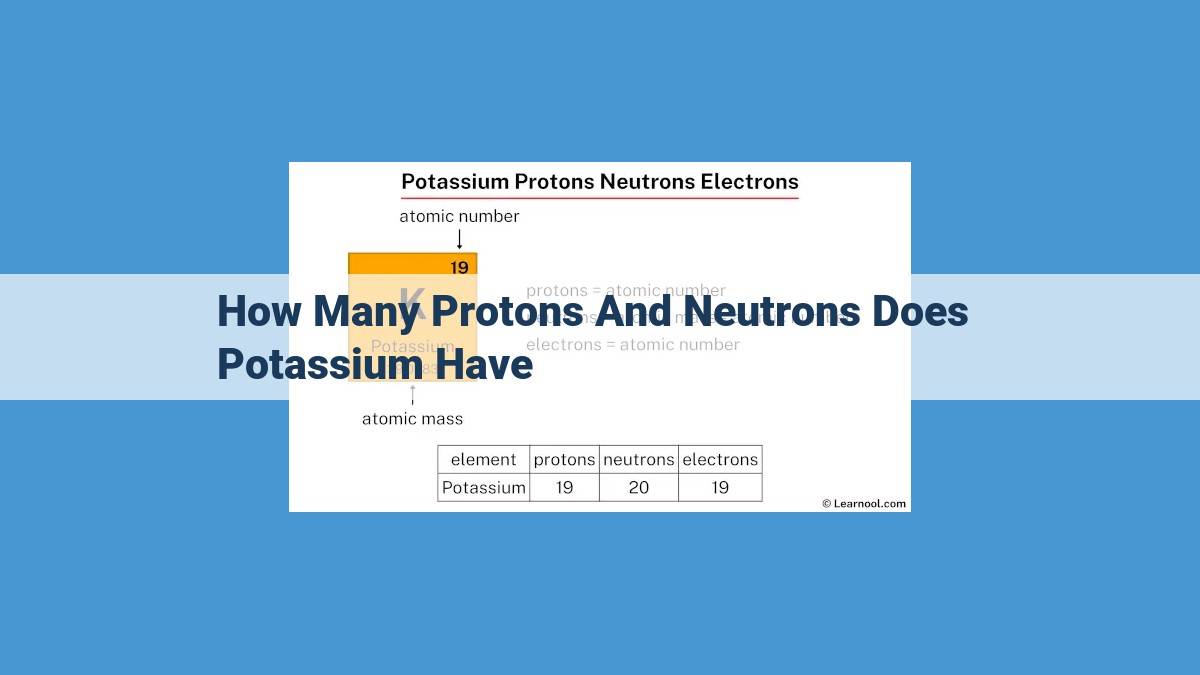
Potassium, with an atomic number of 19, has 19 protons in its nucleus. Its atomic mass of 39.1 indicates an average of approximately 20 neutrons, as different stable isotopes of potassium exist with varying neutron numbers. The relative abundance of these isotopes determines the weighted average neutron count. Understanding the number of protons and neutrons in potassium is crucial for comprehending its chemical properties and its significance in biological processes.
Unveiling the Secrets of Potassium: A Journey into Atomic Composition
In the fascinating realm of chemistry, understanding the atomic composition of elements is paramount. It’s like deciphering the building blocks of our universe, unraveling the secrets that govern their behavior and interactions. One such element that has captivated scientists and intrigued curious minds alike is potassium. Join us on an adventure as we delve into the atomic composition of potassium, uncovering the number of protons and neutrons that define its unique identity.
The Significance of Atomic Composition
Every element in the periodic table, from the tiniest hydrogen to the mighty uranium, possesses a distinctive atomic composition. This composition is determined by the number of protons, neutrons, and electrons within its atoms. Protons, found in the nucleus of the atom, carry a positive charge and dictate the element’s identity. Neutrons, also residing in the nucleus, lack a charge and contribute to the overall mass of the atom. Understanding the number of protons and neutrons in an element is crucial for predicting its chemical properties and behavior.
Purpose of this Exploration
Our quest today focuses on determining the number of protons and neutrons in potassium. This remarkable element, adorned with the symbol K, plays a pivotal role in various biological processes, including nerve function and muscle contraction. By unraveling its atomic composition, we can gain deeper insights into its significance and how it influences our world.
Atomic Number and Protons in Potassium: A Story of Identity and Structure
Within the vast tapestry of the chemical world, each element holds a unique identity, determined by its atomic number. This number, akin to a fingerprint, reveals the essence of an element and distinguishes it from all others. For potassium, this atomic fingerprint bears the number 19, a number that holds profound implications for the element’s innermost structure.
Imagine a tiny atom of potassium, a microscopic universe teeming with subatomic particles. At its core lies the nucleus, a dense, positively charged realm home to two fundamental particles: protons and neutrons. Protons, the bearers of positive charge, play a pivotal role in defining an element’s identity. Each element possesses a unique number of protons, and it is this number that determines its position on the periodic table, the chemist’s roadmap to the elements.
For potassium, the atomic number of 19 signifies that each atom of this element harbors 19 protons. These protons, like tiny magnets, create a positive charge within the nucleus. The number of protons is so fundamental to an element’s identity that it cannot be altered without fundamentally changing the element itself. It is the unwavering signature that distinguishes potassium from all other elements.
Atomic Mass and Neutrons in Potassium: Unraveling the Enigma
In the realm of chemistry, deciphering the secrets of atomic composition is akin to unlocking a treasure trove of knowledge. Amidst the array of elements that grace the periodic table, potassium stands out as a captivating subject of investigation, inviting us to delve into its atomic makeup.
The concept of atomic mass is central to understanding the number of neutrons within an element. Atomic mass, expressed in atomic mass units (amu), represents the average mass of an element’s atoms. This value is not a whole number due to the existence of isotopes, atoms of the same element with varying numbers of neutrons.
Potassium boasts three prominent stable isotopes:
-
Potassium-39: The most abundant isotope, accounting for about 93% of natural potassium. It possesses 19 protons and 20 neutrons, yielding an atomic mass of 39.0983 amu.
-
Potassium-40: A radioactive isotope with a percentage of about 0.01%. It contains 19 protons and 21 neutrons, resulting in an atomic mass of 39.9639 amu.
-
Potassium-41: A rare isotope, contributing less than 1% to the total. It comprises 19 protons and 22 neutrons, with an atomic mass of 40.9618 amu.
Each isotope contributes to the overall weighted average atomic mass of potassium. By multiplying the atomic mass of each isotope by its relative abundance and summing them up, we arrive at the average atomic mass:
Potassium’s weighted average atomic mass = ((0.93 * 39.0983 amu) + (0.01 * 39.9639 amu) + (0.001 * 40.9618 amu)) = 39.102 amu
Given that the average atomic mass is approximately 39.1 amu, it suggests that on average, potassium atoms contain about 20 neutrons. This value helps us deduce the neutron number of potassium atoms, which plays a crucial role in determining their behavior in chemical reactions and biological processes.
Weighted Average and Neutron Abundance in Potassium
Understanding the atomic composition of elements is crucial in chemistry, as it determines their chemical behavior and interactions with other substances. In this blog post, we will embark on a quest to determine the number of protons and neutrons in potassium, an element essential for biological systems.
Atomic Mass and the Neutron Count
Every element is characterized by its atomic number, which represents the number of protons in its nucleus. Potassium’s atomic number is 19, indicating that it has 19 protons. However, atoms also contain neutrons, which contribute to the overall mass of the atom without altering its charge.
Stable Isotopes and Neutron Abundance
Potassium exists in three stable isotopes: potassium-39, potassium-40, and potassium-41. These isotopes have the same number of protons (19) but differ in the number of neutrons. Potassium-39 has 20 neutrons, potassium-40 has 21 neutrons, and potassium-41 has 22 neutrons.
Weighted Average Atomic Mass
The atomic mass of an element is a weighted average that takes into account the relative abundance of its isotopes. The weighted average atomic mass of potassium is approximately 39.1. This value suggests that the majority of potassium atoms exist as potassium-39.
Estimating the Average Neutron Abundance
To estimate the average number of neutrons in potassium atoms, we can use the weighted average atomic mass and the numbers of neutrons in each isotope.
Average number of neutrons ≈ Weighted average atomic mass - Atomic number
Average number of neutrons ≈ 39.1 - 19
Average number of neutrons ≈ 20
Therefore, the average potassium atom has approximately 20 neutrons.
Through our exploration, we have determined that potassium has 19 protons and approximately 20 neutrons. This information is essential for understanding potassium’s chemical behavior, as the number of protons determines its charge and the number of neutrons influences its mass and stability. As we continue to uncover the atomic composition of elements, we deepen our understanding of the building blocks of our world.