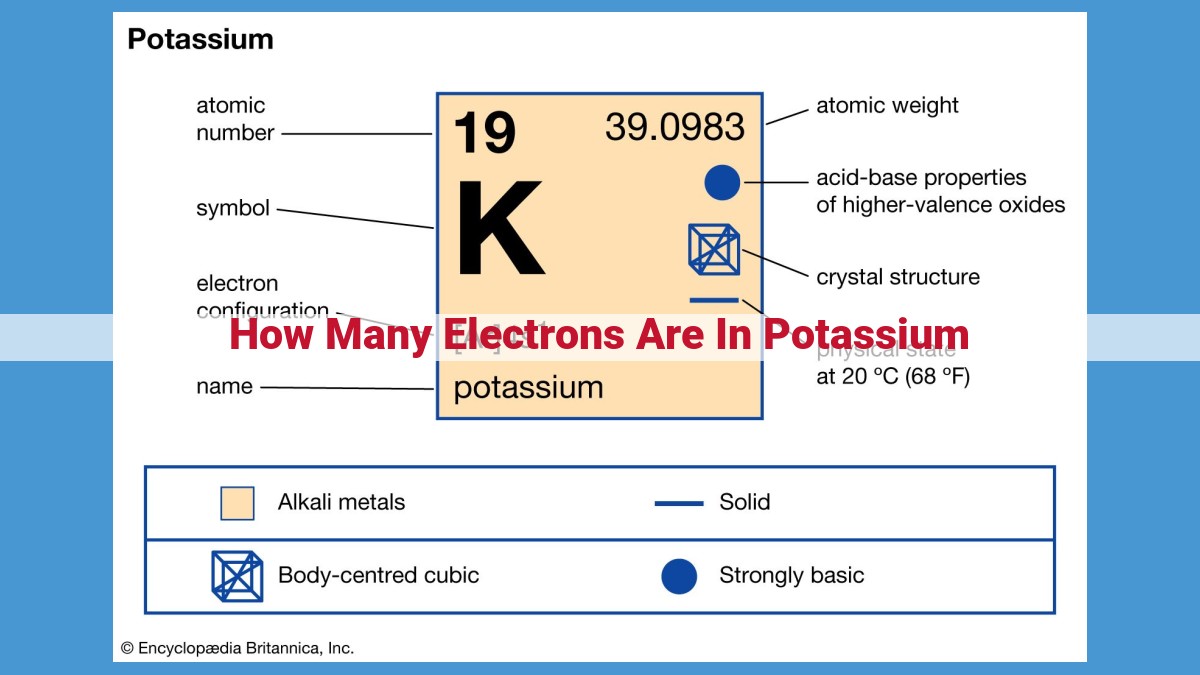
Potassium, an alkali metal with atomic number 19, possesses 19 electrons. This number corresponds to the number of positively charged protons in its nucleus. As the number of protons determines an element’s identity, potassium’s 19 protons define it as the 19th element. In neutral atoms, the number of electrons balances the number of protons, resulting in an equal number of positive and negative charges, ensuring electrical neutrality. The presence of 19 electrons in potassium plays a crucial role in its chemical properties, particularly its single valence electron, which governs its reactivity and biological significance in processes like nerve impulse transmission.
Potassium: The Essential Alkali Metal
Potassium, like a beacon of stability, stands among the alkali metals, a group of elements known for their exceptional reactivity and metallic shine. These elements share a common trait: a single, loosely bound electron in their outermost energy level. This electron, the valence electron, makes alkali metals highly reactive and eager to form bonds with other atoms.
Potassium, with an atomic number of 19, holds a special place in this family. It’s the sixth most abundant element in the Earth’s crust, and its vital role in biological processes makes it an essential nutrient for all living organisms.
In our bodies, potassium reigns supreme as the primary intracellular cation, transcending cell membranes with ease. This movement of potassium across membranes maintains electrical gradients, ensuring the proper functioning of nerve impulses and heart contractions. It also plays a crucial role in muscle contraction, acid-base balance, and cellular water balance.
Atomic Number of Potassium: 19
- Concept of atomic number
- Significance of atomic number in identifying elements
Atomic Number: The Unique Identifier of Elements
In the realm of chemistry, the atomic number holds immense significance, serving as a unique fingerprint that distinguishes one element from another. Each element possesses a characteristic atomic number, a numerical value that represents the number of protons within its nucleus. Protons, being positively charged particles, play a pivotal role in determining the fundamental properties of an element.
Atomic Number Unveils Elemental Identity
The atomic number of an element is a crucial piece of information for scientists, as it provides the key to identifying the element. Every known element has a unique atomic number, allowing chemists to classify and categorize them systematically. The Periodic Table of Elements, a cornerstone of chemistry, is organized based on atomic numbers, with elements arranged in order of increasing atomic number.
Protons: The Cornerstone of Electrical Balance
Protons, along with neutrons and electrons, are the fundamental building blocks of atoms. Protons and neutrons reside in the atom’s nucleus, while electrons orbit around the nucleus. The number of protons in an atom dictates its electrical charge. Since protons carry a positive charge and electrons carry a negative charge, a neutral atom contains an equal number of protons and electrons. This balance is essential for the stability and chemical behavior of an element.
Potassium: Spotlight on an Essential Element
Potassium, an element with the symbol K, is a prime example of how atomic number influences an element’s properties and behavior. Potassium possesses an atomic number of 19, indicating that its nucleus contains 19 protons. This atomic number places potassium in Group 1 of the Periodic Table, known as the alkali metals. Alkali metals are highly reactive due to the presence of a single valence electron, which is loosely bound to the nucleus.
Potassium’s Role in Biological Processes
Potassium’s valence electron plays a critical role in its involvement in various biological processes. As a vital electrolyte, potassium helps regulate fluid balance and supports nerve and muscle function. It participates in numerous cellular mechanisms, including the transmission of nerve impulses and the contraction of muscles. The human body requires a steady supply of potassium for optimal health and well-being.
The atomic number serves as a cornerstone of chemistry, enabling scientists to identify and understand the elements that make up our world. By understanding the atomic number of potassium, we gain insights into its unique properties and its essential role in biological systems.
Number of Protons in Potassium: 19
In the realm of chemistry, potassium stands out as an essential element with numerous fascinating properties. To delve into its intricate nature, we must understand the concept of protons and their crucial role in shaping potassium’s identity.
Atomic Number and Protons: An Intimate Bond
Each element in the periodic table is assigned a unique atomic number, a number that plays a pivotal role in understanding the element’s characteristics. The atomic number represents the number of protons residing in the element’s nucleus. In the case of potassium, its atomic number is 19, indicating that each potassium atom holds 19 protons within its nucleus.
Protons: The Guardians of Electrical Balance
Protons carry a positive charge, a fundamental property that significantly influences the atom’s overall electrical neutrality. The number of protons in an atom must balance the number of negatively charged electrons to maintain a neutral state. Therefore, the number of protons in potassium, 19, ensures that the atom remains electrically neutral, with 19 electrons orbiting the nucleus.
This delicate balance between protons and electrons is crucial for potassium’s stability and its ability to interact with other elements. Protons provide the foundation for potassium’s chemical properties, enabling it to form bonds and participate in various chemical reactions.
In summary, the number of protons in potassium, 19, plays a fundamental role in the element’s atomic structure, electrical neutrality, and chemical reactivity. By understanding the intimate relationship between atomic number and protons, we gain deeper insights into the fascinating world of potassium and its vital contributions to various biological processes.
Number of Electrons in Potassium: 19
- Concept of electrons and their role in atoms
- Equal number of electrons and protons in neutral atoms
- Valence electron in potassium and its chemical significance
The Significance of Electrons in Potassium’s Chemistry
In the realm of chemistry, understanding the electronic structure of elements is crucial for deciphering their properties and functions. Potassium, an essential alkali metal, exemplifies this concept.
Electrons, the negatively charged particles that orbit an atom’s nucleus, play a profound role in determining its behavior. In a neutral atom, the number of electrons is precisely equal to the number of protons within its nucleus. This equilibrium ensures electrical neutrality.
Valence Electrons: The Key to Chemical Reactivity
Among the electrons in potassium, the valence electron holds particular significance. This electron, located in the outermost energy level, is responsible for chemical bonding. Its presence determines potassium’s reactivity and ability to form compounds.
In the case of potassium, it possesses a single valence electron. This solitary electron is eager to participate in chemical reactions, creating a strong tendency for potassium to form positive ions. This ionic character is essential for many biological processes and industrial applications.
The Importance of Potassium in Biological Systems
Potassium is a vital component of living organisms, playing a crucial role in maintaining cell function, nerve impulses, and fluid balance. Its involvement in these processes underscores the significance of its electronic structure.
The presence of a single valence electron facilitates the formation of potassium ions, which are essential for the electrical excitability of cells. This ability underlies nerve impulses, muscle contractions, and cell communication.
Furthermore, potassium ions are involved in the maintenance of fluid balance across cell membranes. By establishing a concentration gradient, potassium helps regulate water movement, ensuring proper cell hydration and function.
Potassium’s atomic structure, with its specific number of electrons, has profound implications for its chemical properties and biological functions. The valence electron, in particular, plays a pivotal role in potassium’s reactivity and its crucial role in living organisms. Understanding the electronic structure of elements like potassium is essential for appreciating the intricate workings of the chemical world and the diverse ways in which elements shape our lives.
Summary
- Recap of the key points discussed
- Importance of potassium’s electronic structure in its properties and biological functions
Potassium: A Vital Element with a Unique Electronic Structure
In the vast realm of elements, potassium stands out as a crucial component of life. Let’s delve into its atomic structure to uncover the secrets behind its remarkable properties, starting with its classification as an alkali metal.
Alkali metals, including potassium, are known for their high reactivity and distinctive characteristics. They readily lose their outermost electron, forming cations with a +1 charge. This ability grants them a silvery-white appearance and makes them excellent conductors of electricity and heat.
Potassium holds the atomic number 19, indicating the number of protons within its nucleus. Protons are positively charged particles that determine an element’s identity. Potassium’s atomic number distinguishes it from any other element, making it unique on the periodic table.
What’s equally intriguing is potassium’s number of protons. It possesses 19 protons, matching its atomic number, since atoms maintain electrical neutrality by balancing positively charged protons with negatively charged electrons.
Complementing the protons, potassium has an equal number of electrons, also 19. Electrons reside in orbitals surrounding the nucleus, arranged in distinct energy levels. The valence electron, located in the outermost energy level, determines an element’s chemical behavior.
Potassium’s single valence electron makes it highly reactive. It readily forms bonds with other atoms to attain a stable electronic configuration. This reactivity is essential for potassium’s role in numerous biological processes.
For instance, potassium plays a crucial role in maintaining proper fluid balance across cell membranes, enabling essential functions such as nerve transmission and muscle contraction. Additionally, it aids in regulating heart rhythm and blood pressure.
In conclusion, potassium’s unique electronic structure—with its alkali metal nature, atomic number of 19, and lone valence electron—underpins its remarkable properties and indispensable biological functions. Understanding this atomic structure empowers us to appreciate the vital importance of this element in our lives.