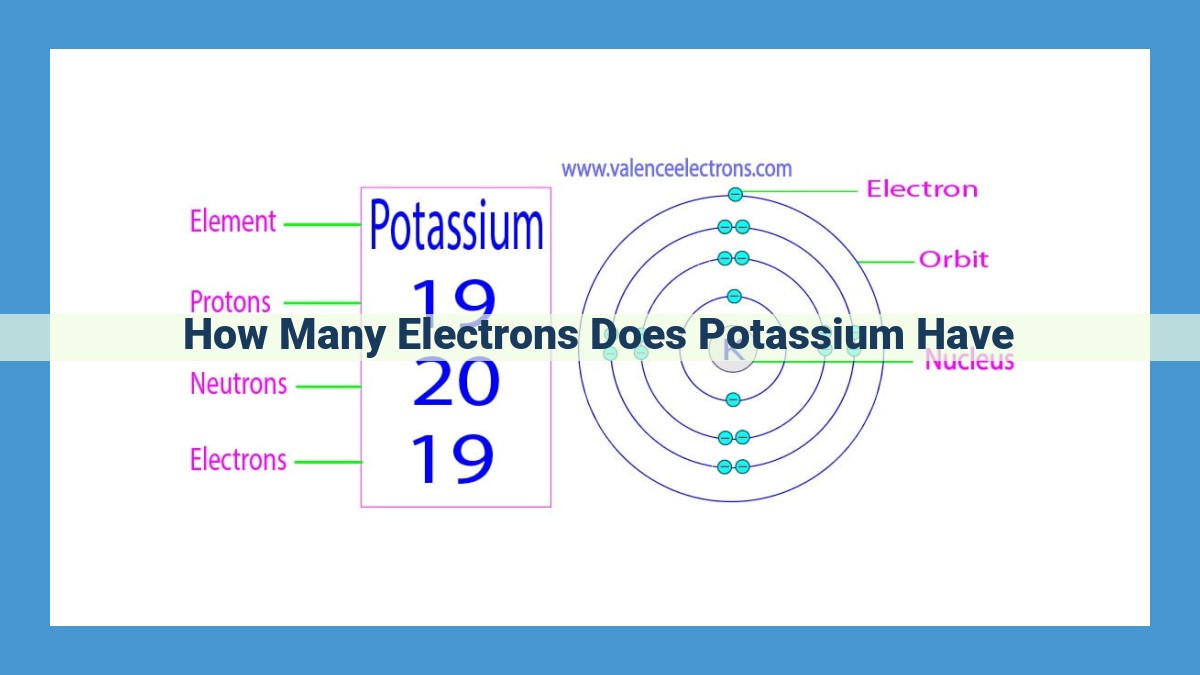
The electron configuration of potassium can be determined through quantum mechanics. Potassium’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹, indicating it has 19 electrons. These electrons occupy four energy levels or shells, with two electrons in the first shell, eight in the second and third shells, and one in the fourth shell. The valence electrons in potassium are the one electron in the fourth shell, which determines its chemical reactivity. Potassium’s atomic number, 19, represents the number of protons and electrons in its neutral state.
Potassium’s Electronic Configuration: A Tale of Subatomic Symphony
Potassium, an alkali metal with the atomic number 19, stands out in the periodic table for its remarkable electron configuration. To understand this configuration, let’s embark on a journey into the world of quantum mechanics and subatomic particles.
Electron Configuration and Quantum Numbers
Every electron orbiting an atom occupies a specific quantum state, characterized by four quantum numbers: principal, azimuthal, magnetic, and spin. The principal quantum number (n) represents the electron’s energy level, with electrons in higher energy levels having greater distance from the nucleus. The azimuthal quantum number (l) describes the shape of the electron’s orbital, with different values corresponding to s, p, d, and f orbitals.
Potassium’s Electron Configuration
Potassium’s electron configuration is written as 1s² 2s² 2p⁶ 3s¹. This notation reveals that potassium has:
- Two electrons in the first energy level (n=1), occupying the 1s orbital.
- Eight electrons in the second energy level (n=2), occupying the 2s and 2p orbitals.
- One electron in the third energy level (n=3), occupying the 3s orbital.
Visualizing Potassium’s Electron Arrangement
We can illustrate potassium’s electron configuration using diagrams called orbital diagrams. These diagrams show the spatial arrangement and energy of electrons within an atom. For potassium, the orbital diagram resembles a tetrahedron, with the 3s electron being unpaired.
In conclusion, understanding potassium’s electron configuration is crucial for comprehending its chemical behavior. The arrangement of electrons in potassium’s orbitals determines its valence and oxidation states, making it an essential property for understanding its reactivity with other elements.
The Quantum World: Unraveling the Number of Electron Shells in Potassium
In the realm of atoms, the electrons dance around the nucleus, each occupying specific energy levels known as electron shells. The enchanting waltz of these electrons plays a pivotal role in shaping the atom’s properties and behavior.
Potassium, an alkali metal with atomic number 19, captivates our attention today. As we venture into the quantum realm, let’s uncover the mysteries surrounding the number of electron shells in this fascinating element.
The concept of electron shells arises from quantum mechanics. This intriguing theory describes the electrons as occupying well-defined energy states. The shells, or energy levels, are analogous to the orbits of celestial bodies, each shell accommodating a set number of electrons.
The energy of each shell increases as we move away from the nucleus. This means that electrons in higher shells possess more energy compared to those in lower shells. The shell model of the atom visualizes these energy levels as concentric circles surrounding the nucleus, with electrons filling these shells from the innermost outward.
Potassium, with its 19 electrons, fills its shells in the following manner:
-
1st Shell (K shell): This innermost shell, closest to the nucleus, can accommodate a maximum of 2 electrons. In potassium, this shell is filled, housing 2 electrons.
-
2nd Shell (L shell): The second shell, slightly farther from the nucleus, can hold up to 8 electrons. Potassium’s second shell is also filled, containing 8 electrons.
-
3rd Shell (M shell): The outermost shell in potassium, the third shell, can accommodate a maximum of 18 electrons. In potassium, this shell is partially filled, containing 9 electrons.
In summary, potassium has 3 electron shells, with 2 electrons in the K shell, 8 electrons in the L shell, and 9 electrons in the M shell. This unique electron configuration gives potassium its distinctive chemical properties and reactivity, making it an essential element in various biological processes and industrial applications.
Valence Electrons in Potassium: A Key Factor in Chemical Bonding
In the realm of chemistry, the electronic structure of an element plays a crucial role in dictating its behavior. Valence electrons, in particular, are like the versatile building blocks that determine an element’s ability to form chemical bonds with others.
Potassium, a highly reactive metal in the periodic table, possesses a unique valence electron configuration that makes it a master of bonding. Potassium has one valence electron in its outermost shell, which orbits the nucleus with a remarkable energy level. This lone electron acts as a gateway for potassium to participate in various chemical reactions.
The number of valence electrons also influences an element’s oxidation states. Oxidation states represent the number of electrons an atom can gain or lose to achieve a more stable electronic configuration. For potassium, its single valence electron means it can easily lose this electron to form a positive ion with an oxidation state of +1. This oxidation state is the most common for potassium compounds.
The presence of a single valence electron also explains potassium’s high chemical reactivity. Potassium atoms are eager to shed their valence electron to achieve stability, making them highly electropositive. This electropositivity drives potassium’s tendency to form ionic bonds with elements that have a strong electronegativity, such as chlorine or oxygen.
In summary, potassium’s single valence electron is a key factor in understanding its chemical bonding behavior. This valence electron allows potassium to form stable bonds, participate in various reactions, and exhibit its characteristic electropositivity. By delving into the electronic structure of potassium, we gain insights into the fundamental forces that shape its chemical interactions.
Electronic Structure of Potassium
Journey into the fascinating realm of potassium’s electronic structure and uncover the intricate dance of subatomic particles within this alkali metal.
Potassium, a vital element in our biological systems, possesses a unique arrangement of electrons that shapes its chemical properties and reactivity. To unravel these characteristics, we must delve into the principles of quantum mechanics, the governing force behind the behavior of electrons in atoms.
The overall electronic structure of potassium reflects the precise organization of its electrons within specific energy levels, known as atomic orbitals. These orbitals, depicted by mathematical equations, describe the probable locations where electrons can be found around the atom’s nucleus.
The nucleus, the heart of the atom, houses densely packed protons and neutrons. Protons, positively charged, determine the atomic number of potassium, which plays a pivotal role in identifying its place in the periodic table and defining its chemical properties.
Electrons, on the other hand, are negatively charged particles that orbit the nucleus in specific energy levels. The number of electrons in an atom always matches the number of protons, ensuring electrical neutrality.
Potassium has 19 electrons, arranged in concentric electron shells. The first shell, closest to the nucleus, is filled with 2 electrons. The second shell contains 8 electrons, while the third and outermost shell holds the remaining 9 electrons.
The outermost electrons, known as valence electrons, play a crucial role in chemical bonding. Potassium has one valence electron in its outermost shell, making it a highly reactive element that readily forms chemical bonds with other elements to achieve a stable electron configuration.
The arrangement of electrons in potassium’s atomic orbitals is governed by the principles of quantum mechanics. These principles dictate the energy levels that electrons can occupy and the shapes of the atomic orbitals that describe their probable locations.
The electronic structure of potassium provides a blueprint for understanding its chemical behavior and reactivities. It explains why potassium is a highly reactive metal that readily forms ions, giving it its unique properties and applications in various scientific and industrial fields.
The Atomic Number of Potassium: Uncovering the Essence of a Reactive Metal
In the vast tapestry of elements that make up our universe, potassium stands out as a highly reactive metal with unique properties. Its atomic number, a fundamental characteristic that defines its identity, plays a pivotal role in shaping its chemical behavior and distinguishing it from other elements.
The Periodic Table and Atomic Number: A Guiding Map
The periodic table, a tabular arrangement of elements organized by their atomic number, provides a powerful tool for understanding the properties of elements. Atomic number is the number of protons in the nucleus of an atom, which determines the element’s position in the periodic table.
Potassium’s Identity: Atomic Number 19
Potassium, located in Group 1 (alkali metals) and Period 4 of the periodic table, has an atomic number of 19. This means that every potassium atom contains 19 protons in its nucleus. These protons, along with an equal number of electrons, define the atom’s neutral electrical charge.
The Significance of Atomic Number: A Chemical Fingerprint
The atomic number has far-reaching implications for potassium’s chemical properties. It determines the number of valence electrons, which are the outermost electrons in an atom’s orbitals that participate in chemical bonding. Potassium has one valence electron, making it highly reactive and eager to form chemical bonds with other elements.
Furthermore, the atomic number influences the chemical bonding behavior of potassium. Its single valence electron allows it to exhibit +1 oxidation state, indicating its tendency to lose this electron easily during chemical reactions. This characteristic contributes to potassium’s strong reducing power and makes it a valuable reagent in various chemical processes.