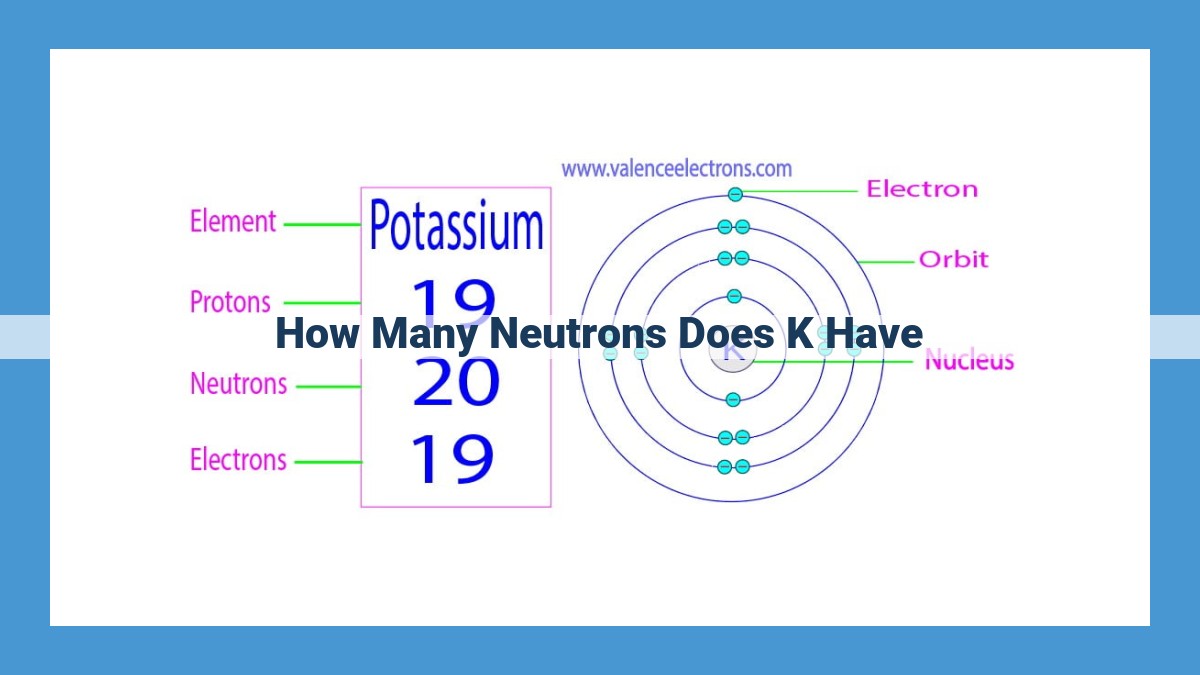
Potassium, with an atomic number of 19, possesses a neutron number of 20. This value is obtained by subtracting the atomic number from the mass number, which is approximately 39. The mass number represents the total number of protons and neutrons in the nucleus. The neutron-to-proton ratio, calculated as 20:19, indicates that potassium’s nucleus contains slightly more neutrons than protons, contributing to its nuclear stability. This neutron abundance plays a crucial role in potassium’s chemical properties and its applications in various fields.
Delving into the Atomic Realm: Unveiling the Secrets of Potassium
Embark on a captivating scientific journey as we explore the enigmatic world of atomic structure and uncover the mysteries surrounding potassium. Let’s delve into the fundamental concepts and learn the remarkable story of this essential element.
Understanding Atomic Structure
Atoms, the building blocks of all matter, consist of a central nucleus and orbiting electrons. The nucleus houses protons (positively charged) and neutrons (neutral), while electrons (negatively charged) dance around the nucleus. Each element is defined by its unique atomic number, which represents the number of protons in its nucleus.
Introducing Potassium: A Vital Element
Potassium, an alkali metal, plays a crucial role in our bodies, regulating fluid balance and maintaining healthy nerve function. With an atomic number of 19, potassium ranks among the most abundant elements on Earth.
Determining Potassium’s Neutron Number
The neutron number of an element represents the number of neutrons in its nucleus. To calculate this number, we subtract the atomic number from the mass number, which is the sum of protons and neutrons.
Calculating Potassium’s Mass Number
The mass number of potassium is 39. This means that each potassium atom contains 39 particles in its nucleus – 19 protons (atomic number) and 39 – 19 = 20 neutrons.
Understanding the Neutron-to-Proton Ratio
The neutron-to-proton ratio is a crucial factor in nuclear stability. Elements with a balanced ratio tend to be more stable than those with significant imbalances. For potassium, we calculate this ratio as 20 (neutron number) / 19 (atomic number) = 1.05.
Determining the Neutron Number in Potassium: A Journey to the Heart of the Atom
In the realm of chemistry, understanding the basic structure of atoms is crucial. Protons, with their positive charge, neutrons, devoid of charge, and electrons, with their negative charge, form the foundation of all matter. Potassium, an element with an atomic number of 19, occupies a special place in this atomic landscape.
To determine the neutron number in potassium, we embark on a scientific quest. The neutron number, represented by the symbol “N,” is the number of neutrons nestled within the atom’s nucleus. It is calculated by subtracting the atomic number (“Z”) from the mass number (“A”). The mass number, in turn, is the sum of protons and neutrons.
[Formula]
N = A – Z
Let’s unravel the mystery of potassium’s neutron number. The element resides in row 4, group 1 of the periodic table. Atomic number indicates the number of protons, which in this case is 19. Potassium’s mass number is 39, as stated in its elemental symbol (“K”39). Substituting these values into our formula, we get:
N = 39 – 19
N = 20
Potassium, therefore, boasts 20 neutrons whirling within its nucleus. This neutron number plays a pivotal role in the atom’s stability and its participation in various chemical reactions. Understanding this concept empowers us with a deeper appreciation of the atomic world and the intricacies that govern the elements around us.
Calculating the Mass Number of Potassium: Unlocking the Secrets of the Nucleus
In the realm of atoms, potassium stands out as an enigmatic element, harboring secrets within its nucleus. To unravel these mysteries, we must embark on a journey to determine its mass number, a fundamental property that reveals the total number of protons and neutrons it contains.
What is Mass Number?
Mass number is a pivotal concept in understanding atomic structure. It represents the sum total of protons and neutrons residing in an atom’s nucleus. Each proton carries a positive charge, while neutrons are neutrally charged. Together, they constitute the atomic nucleus, the heart of the atom.
Mass Number and Atomic Mass
The mass number of an element is intricately linked to its atomic mass, a weighted average of the masses of all its naturally occurring isotopes. Isotopes are variations of an element that possess the same atomic number but differ in the number of neutrons.
Understanding the relationship between mass number and atomic mass is crucial. Atomic mass is not an integer, as it incorporates the contributions of different isotopes. However, for most practical purposes, the mass number provides a close approximation of the atom’s actual mass.
Calculating Potassium’s Mass Number
Potassium, with an atomic number of 19, has several isotopes. The most abundant isotope, known as potassium-39, has a mass number of 39. This indicates that the nucleus of potassium-39 contains 19 protons (the atomic number) and 20 neutrons (the difference between mass number and atomic number).
Significance of Mass Number
The mass number of an element provides valuable insights into its nuclear stability and properties. It helps scientists predict radioactive decay patterns, nuclear reactions, and the behavior of atoms in chemical reactions. Understanding the mass number allows us to unravel the secrets of the atomic world and harness its power for technological advancements.
Finding the Atomic Number of Potassium
- Define atomic number as the number of protons in the nucleus.
- Determine the atomic number of potassium based on its location on the periodic table.
Unveiling the Atomic Number of Potassium: A Journey to the Periodic Table
In the realm of chemistry, understanding the intricacies of atomic structure is paramount. One such element, potassium, holds a unique place in our understanding of these building blocks of matter. In this exploration, we embark on a journey to unravel the secrets of potassium’s atomic number, a fundamental property that defines its very essence.
The Genesis of Atomic Number
An atom’s nucleus, its heart, harbors protons, positively charged particles. The atomic number, a characteristic fingerprint of each element, reflects the number of protons residing within this nucleus. This number serves as a cornerstone in characterizing elements and their position on the periodic table.
Potassium’s Place on the Periodic Tableau
The periodic table, a symphony of organized elements, provides a roadmap to potassium’s atomic number. As we navigate its rows and columns, we encounter potassium tucked away in Group 1, a collection of elements known as alkali metals. Its symbolic representation, K, hints at the 19 protons that define its identity.
Unlocking the Atomic Number’s Significance
The atomic number holds immense significance in shaping an element’s properties. It dictates the number of electrons orbiting the nucleus, in turn, influencing the element’s chemical behavior and reactivity. For instance, potassium’s single valence electron makes it highly reactive, giving rise to its role in biological processes and industrial applications.
Our journey has unveiled the atomic number of potassium, an element that has captured the attention of scientists and industrialists alike. Its 19 protons form the core of its identity, guiding its chemical interactions and unlocking its potential in various fields. Understanding the atomic number not only enriches our knowledge of potassium but also opens the door to a deeper appreciation of the complexities of the atomic world.
Unveiling the Mystique of Potassium: Its Neutron-to-Proton Ratio
In the captivating realm of atomic science, where the secrets of matter unfold, we embark on a journey to unravel the mysteries of potassium. This enigmatic element holds a profound significance in our world, and today, we delve into one of its quintessential attributes: the neutron-to-proton ratio.
At the heart of every atom lies a central nucleus, a bustling metropolis teeming with protons, the positively charged inhabitants, and neutrons, their uncharged counterparts. The interplay between these fundamental particles dictates the stability of the atom, influencing its behavior and shaping its destiny.
The neutron-to-proton ratio, a vital indicator of nuclear stability, quantifies the relative abundance of neutrons to protons within the nucleus. This ratio plays a pivotal role in determining whether an atom will succumb to radioactive decay, a process that transforms it into a more stable state.
Calculating Potassium’s Neutron-to-Proton Ratio
To unravel the intricacies of potassium’s neutron-to-proton ratio, we embark on a scientific adventure. We begin by identifying the atomic number of potassium, which stands at 19. This number represents the number of protons harbored within its nucleus.
Next, we delve into the realm of mass number. This enigmatic figure encapsulates the total count of protons and neutrons nestled within the nucleus. For potassium, its mass number is 39.
Equipped with these crucial pieces of information, we can now unveil the neutron number: the enigmatic complement to the atomic number. This elusive quantity is calculated by deftly subtracting the atomic number from the mass number. For potassium, this subtraction yields a neutron number of 20.
Finally, we arrive at the crux of our quest: the neutron-to-proton ratio. This enigmatic figure is derived by dividing the neutron number by the atomic number. In the case of potassium, this calculation reveals an intriguing ratio of 1.05.
The Significance of Potassium’s Neutron-to-Proton Ratio
The neutron-to-proton ratio unveils a hidden truth about potassium’s nuclear stability. This ratio of 1.05 indicates a relatively balanced interplay between protons and neutrons, contributing to the stability of the potassium nucleus.
In contrast, atoms with significantly disparate neutron-to-proton ratios tend to exhibit unstable behavior, often undergoing radioactive decay to achieve a more harmonious balance. This process, driven by the relentless pursuit of nuclear stability, shapes the very fabric of the atomic world.
Our journey to unravel the mysteries of potassium’s neutron-to-proton ratio has led us to uncover a fundamental aspect of its nuclear identity. This ratio, standing at 1.05, unveils a stable nucleus that defies radioactive decay and ensures potassium’s enduring presence in the elemental landscape. As we delve deeper into the intricacies of the atomic realm, the neutron-to-proton ratio emerges as a beacon of stability, guiding our understanding of the forces that govern the very essence of matter.