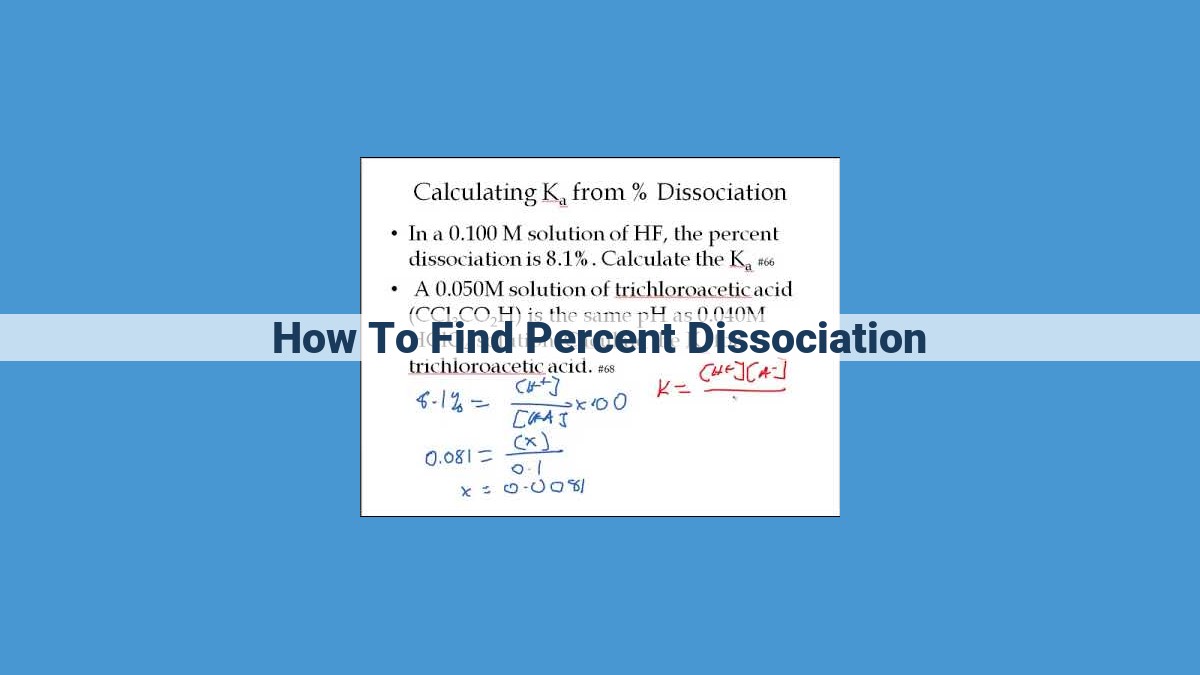
Percent dissociation quantifies the degree of dissociation of a compound in an equilibrium reaction. To calculate it, determine the equilibrium concentration of dissociated ions using an ICE table. The percent dissociation is then calculated as the ratio of the equilibrium concentration of dissociated ions to the initial concentration of the compound, multiplied by 100%. Factors like equilibrium constant, initial concentration, and temperature influence the extent of dissociation, with a higher equilibrium constant, lower initial concentration, and higher temperature promoting greater dissociation.
Understanding the Fundamentals of Equilibrium Reactions
For those of us fascinated by the intricate dance of chemical reactions, the concept of equilibrium holds a particular allure. Equilibrium occurs when a chemical reaction has reached a state of balance, where the forward and reverse reactions proceed at an equal pace. Understanding equilibrium reactions is essential for grasping the dynamics of many chemical processes.
In order to fully comprehend equilibrium reactions, we must first establish a firm grasp of some key concepts.
- Chemical Equation: An equation that depicts the reactants and products involved in a chemical reaction.
- Equilibrium Constant: A value that represents the ratio of concentrations of products to reactants at equilibrium. It is a constant value for a given reaction at a specific temperature.
- Initial Concentration: The concentration of reactants or products at the start of a reaction.
- Dissociation Constant: For weak acids or bases, it is the equilibrium constant for the dissociation reaction.
- Change in Concentration: The difference between the initial concentration and the equilibrium concentration of a reactant or product.
The *ICE table* serves as a powerful tool for tracking the changes in concentrations over the course of a reaction. ICE stands for Initial, Change, Equilibrium, and as the name implies, the table lists the initial concentrations, changes in concentrations, and equilibrium concentrations for each species involved in the reaction.
Calculating Percent Dissociation: Unraveling the Equilibrium Mystery
In the realm of chemistry, understanding equilibrium reactions is akin to deciphering a captivating puzzle. Among the many parameters that govern these reactions, percent dissociation plays a crucial role. It’s a measure of the extent to which a substance breaks down into its constituent ions. To unravel this enigmatic concept, let’s embark on a journey to explore the formula, steps, and factors that influence percent dissociation.
Unveiling the Formula
The formula for calculating percent dissociation (α) is:
α = [A⁻]/[A₀] x 100%
where:
- [A⁻] represents the equilibrium concentration of dissociated ions.
- [A₀] represents the initial concentration of the substance before dissociation.
This formula reveals the significance of ion concentration and initial concentration in determining percent dissociation. By comparing these values, we can gauge the degree of substance breakdown.
Establishing Equilibrium: A Step-by-Step Guide
Establishing equilibrium is essential for calculating percent dissociation. It involves the following steps:
- Initialize the ICE Table: Construct an ICE (Initial, Change, Equilibrium) table to track the changes in concentrations as the reaction progresses.
- Apply Equilibrium Constant: Use the equilibrium constant (Keq) to establish the equilibrium concentrations of dissociated ions and undissociated molecules.
- Calculate Equilibrium Concentration: Solve for the equilibrium concentration of dissociated ions ([A⁻]) using the ICE table and the equilibrium constant.
Unveiling the Equilibrium Concentration
The equilibrium concentration of dissociated ions is obtained by solving the expression:
[A⁻] = [Keq] x [A₀] / [1 + Keq]
This formula demonstrates how equilibrium constant (Keq) and initial concentration ([A₀]) influence percent dissociation. A higher Keq or a lower [A₀] favors dissociation, leading to a higher equilibrium concentration of dissociated ions.
By grasping these concepts and applying the formula, we can accurately determine percent dissociation and uncover the underlying principles governing equilibrium reactions.
Factors Affecting Percent Dissociation: Unveiling the Hidden Dynamics
Understanding the intricate world of chemical equilibrium is crucial for deciphering the behavior of substances in solution. Among the key parameters that influence equilibrium, the equilibrium constant, initial concentration, and temperature play pivotal roles in shaping percent dissociation, a measure of the extent to which a substance breaks down into its components.
Equilibrium Constant:
The equilibrium constant, denoted by Kc, serves as a barometer of the reaction’s favorability. A higher Kc indicates a greater tendency for reactants to form products, resulting in a higher percent dissociation. Conversely, a lower Kc implies a shift towards reactants, leading to a lower percent dissociation.
Initial Concentration:
The initial concentration of the solute sets the stage for the equilibrium process. By increasing the initial concentration, more solute molecules are available for dissociation, thus driving the reaction towards product formation and consequently increasing the percent dissociation.
Temperature:
Temperature acts as a catalyst, influencing the kinetic energy of molecules. As temperature rises, the molecules gain energy and become more likely to overcome the activation energy barrier, leading to increased percent dissociation. This is especially true for endothermic reactions, which absorb heat to promote product formation.
In essence, these factors work in concert to determine the extent of dissociation. A large equilibrium constant, high initial concentration, and elevated temperature favor higher percent dissociation, while the opposite conditions hinder it. By understanding and manipulating these factors, scientists can harness the power of chemical equilibrium to achieve desired reactions and optimize chemical processes.