Radioactive decay provides a reliable method for dating sedimentary rocks. By utilizing radioisotopes with known decay rates, scientists can determine the age of the rock by measuring the abundance of the parent and daughter isotopes. This direct dating approach relies on the presence of suitable radioisotopes within the rock itself. Alternatively, indirect dating methods utilize minerals or fossils within the rock that have been in radioactive equilibrium with their surroundings, allowing for the estimation of the time since burial. These techniques offer valuable insights into the formation and depositional history of sedimentary rocks, aiding in deciphering the Earth’s complex geologic past.
Understanding Radioactive Decay: Unraveling the Mystery of Elements’ Transformation
Radioactive decay is a fascinating phenomenon that unveils the secrets of unstable atomic nuclei. It’s a process through which certain elements undergo spontaneous disintegration, emitting particles or energy to achieve a more stable configuration. Understanding radioactive decay is crucial for various scientific fields, from geology to medical imaging.
Types of Radioactive Decay
There are three primary types of radioactive decay: alpha, beta, and gamma. Each type involves the release of different particles, leading to unique effects on the atom.
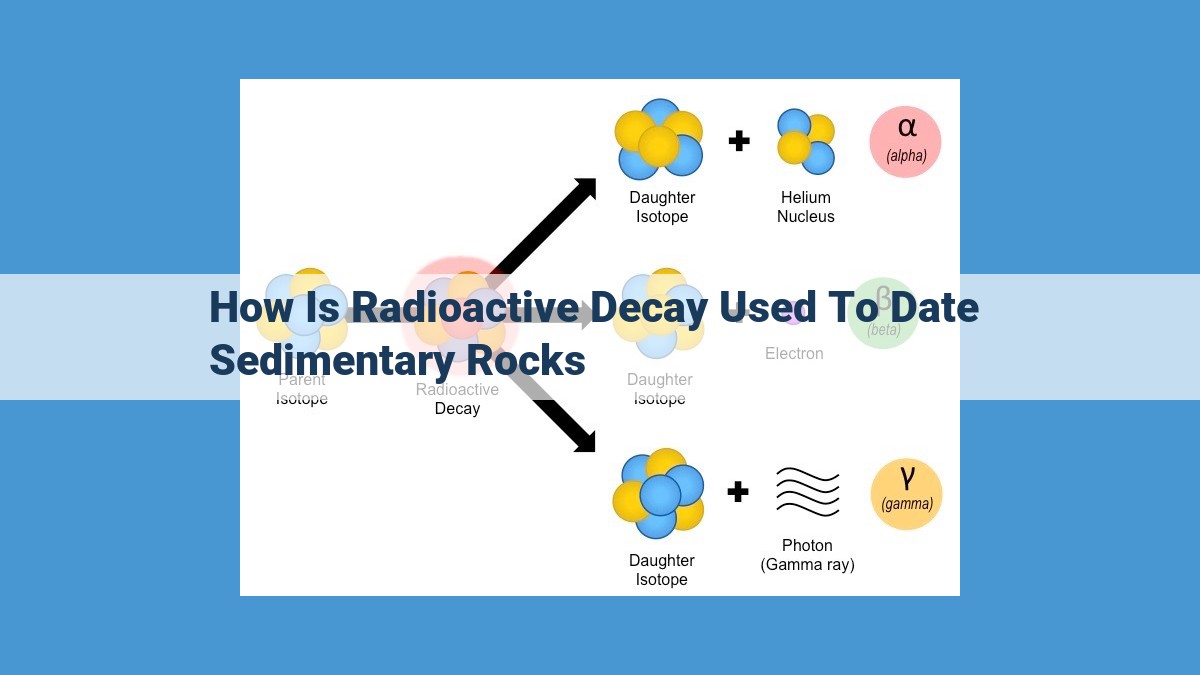
Alpha Decay: In alpha decay, an atom releases an alpha particle, which is essentially a helium nucleus consisting of two protons and two neutrons. The release of an alpha particle decreases the atomic number by 2 and the mass number by 4.
Beta Decay: During beta decay, an atom emits a beta particle, which can be either a beta-minus particle (an electron) or a beta-plus particle (a positron). In beta-minus decay, a neutron in the nucleus is converted into a proton, increasing the atomic number by 1. In beta-plus decay, the opposite occurs, where a proton is converted into a neutron, decreasing the atomic number by 1.
Gamma Decay: Gamma decay involves the emission of high-energy photons or gamma rays. Unlike alpha and beta particles, gamma rays do not affect the atomic number or mass number of the atom. They are released when an excited nucleus transitions to a lower energy state.
Isotopes and Nuclear Stability
- Define isotopes and explain how they differ in stability.
Isotopes and the Dance of Nuclear Stability
In the realm of chemistry and physics, the concept of isotopes holds a pivotal role. Isotopes are variations of the same element, sharing an identical number of protons (positively charged particles in the nucleus), but differing in their number of neutrons (neutral particles in the nucleus).
The stability of an isotope, like a delicate dance, is governed by the balance between neutrons and protons within its nucleus. Stable isotopes possess a harmonious arrangement of neutrons and protons, allowing them to exist indefinitely. In contrast, unstable isotopes, or radioisotopes, possess an imbalance, leading them to undergo radioactive decay, a process in which they transform into a different element.
The stability of an isotope hinges on its neutron-to-proton ratio. Heavy atoms, with a higher number of protons, require more neutrons to maintain a stable equilibrium. If the neutron-to-proton ratio falls out of balance, the nucleus becomes unstable and seeks to restore harmony through radioactive decay.
This quest for stability often involves the emission of alpha particles (helium nuclei) or beta particles (electrons). In the case of alpha decay, the nucleus ejects two protons and two neutrons, effectively reducing its atomic number and mass number by 4. Beta decay, on the other hand, involves the conversion of a neutron into a proton and an electron, thereby increasing the atomic number by 1 while leaving the mass number unchanged.
Understanding isotopes and their nuclear stability is crucial for various scientific disciplines. In geology, for instance, radioactive decay plays a central role in radiometric dating, a technique used to determine the age of rocks and fossils. The decay of specific radioisotopes, such as uranium-238 and carbon-14, provides geologists with a valuable tool to unravel the past.
Moreover, isotopes find applications in medicine, industry, and even archaeology. From using radioisotopes in cancer treatments to tracing the movement of ocean currents, isotopes have become indispensable tools in modern science and technology.
Half-Life and Decay Rate: Understanding the Ticking Clock of Radioactive Elements
In the realm of radioactive elements, there exists a fascinating phenomenon known as radioactive decay. This process, in essence, describes the transformation of an unstable nucleus into a more stable nucleus, releasing particles or energy as a consequence. One cornerstone in the study of radioactive decay is the concept of half-life.
Defining the Half-Life
Half-life is a term that refers to the time it takes for half of the atoms in a radioactive sample to decay. It’s a crucial parameter that helps us understand the rate at which the radioactive element transforms and becomes increasingly stable. Each radioactive element has its unique half-life, ranging from fractions of a second to billions of years.
Relationship between Half-Life and Decay Rate
The half-life of a radioactive element has a direct bearing on its decay rate. Decay rate, expressed in units of atoms decaying per second, measures the speed at which the radioactive substance disintegrates. A shorter half-life corresponds to a faster decay rate, indicating that the element undergoes rapid decay. Conversely, a longer half-life signifies a slower decay rate, suggesting a more gradual transformation process.
Applications of Half-Life and Decay Rate
Half-life and decay rate are invaluable tools used by scientists in various fields, including archaeology, paleontology, and geology. Archaeologists, for instance, utilize radioactive carbon dating to determine the age of ancient artifacts by analyzing the decay rate of carbon-14. Similarly, paleontologists employ radioactive dating techniques to determine the age of fossils and reconstruct past environments. Geologists rely on the decay rate of radioactive elements to measure the age of rocks and decipher the Earth’s history.
Unlocking the Secrets of Time
By unraveling the mysteries of half-life and decay rate, scientists have gained profound insights into the motion of time and the evolution of our planet. These concepts provide a powerful means to unlock the secrets of the past and shed light on the enigmatic tapestry of the Earth’s history.
Index Fossils: Unlocking the Secrets of Rock Layers
In the vast tapestry of Earth’s history, index fossils emerge as invaluable clues, helping us understand the intricate relationships between the different layers of the planet’s crust. These exceptional fossils are the remains of organisms that lived during a specific geological period. Their presence in rock layers allows us to correlate these layers, determining their relative age and understanding the sequence of events that have shaped our planet over eons.
Index fossils are particularly useful in sedimentary rocks, which form when sediments—such as sand, gravel, and mud—accumulate over time. These rocks often contain fossils of marine organisms that lived in the ancient oceans. By comparing the fossils found in different sedimentary layers, geologists can determine which layers were deposited at the same time, even if they are now separated by vast distances.
The key to using index fossils for correlation is their limited geological range. This means that a particular index fossil is only found in rocks that formed during a specific period of time. For example, the trilobite fossil is an excellent index fossil for the Paleozoic Era. Trilobites were marine arthropods that flourished for over 250 million years, but they became extinct at the end of the Paleozoic Era. By identifying trilobite fossils in a rock layer, geologists can confidently conclude that the layer was deposited during the Paleozoic Era.
The use of index fossils in biostratigraphy, the study of the distribution of fossils in rock layers, has revolutionized our understanding of Earth’s history. By correlating rock layers based on their index fossils, geologists can reconstruct past environments, track the evolution of life forms, and unravel the complex geological processes that have shaped our planet.
Unconformities and Geological Structures: Unlocking the Secrets of Time
Deep within the Earth’s crust lies a hidden chronicle of our planet’s astonishing history, preserved in layers of rock and sediment. These layers, known as strata, tell tales of past environments, ancient life forms, and tectonic upheavals. Scattered throughout these strata are enigmatic gaps and interruptions called unconformities.
Unconformities, like missing pages in a book, represent periods of time when no new sediment was deposited or when existing layers were eroded away. They can reveal dramatic events such as mountain-building episodes, sea-level fluctuations, or even meteorite impacts. By studying unconformities, geologists can piece together the timeline of Earth’s geological past.
Accompanying these unconformities are geological structures—faults, folds, and domes—that have deformed the strata. These structures provide clues about the forces that have shaped the Earth’s surface over time. Faults, breaks in the rock, indicate past movements and earthquakes. Folds reveal compression or stretching of the Earth’s crust, while domes suggest the presence of underlying magma or salt.
Together, unconformities and geological structures serve as ** Rosetta stones** of Earth’s history. By deciphering their meaning, geologists can reconstruct ancient landscapes, track the movements of tectonic plates, and uncover the sequence of events that have formed our planet.
Cross-Cutting Relationships: Unraveling the Age Enigma of Rocks
In the realm of geology, determining the relative ages of rocks is crucial to reconstructing the intricate tapestry of Earth’s history. One invaluable technique employed by geologists is the analysis of cross-cutting relationships, which can illuminate the chronological sequence of geological events.
Imagine a geological expedition amidst a rugged terrain. A keen observer notices intersecting veins of igneous rock intruding into layers of sedimentary rock. Cross-cutting relationships refer to the scenarios where a younger feature, such as an igneous intrusion, intersects an older one, such as a sedimentary layer.
The principle underpinning cross-cutting relationships is straightforward: the younger feature must have formed after the older one. In our example, the igneous intrusion sliced through the sedimentary layer after the latter had been deposited and solidified. This deduction is based on the premise that the molten igneous rock would have melted or deformed the sedimentary rock if it had intruded before its solidification.
By examining cross-cutting relationships, geologists can piece together a chronological sequence of events that shaped the geological landscape. Like chapters in a captivating novel, each cross-cutting relationship provides a clue, allowing us to unravel the history of the region, one layer at a time.
Stratigraphy and Rock Layers: Unraveling Earth’s History
In the vast tapestry of geological time, rock layers serve as silent witnesses to the Earth’s tumultuous past. Stratigraphy, the study of these layers, offers a captivating journey into the evolution of our planet.
Sediments, the building blocks of sedimentary rocks, originate from various sources—debris from erosion, remains of living organisms, and chemical precipitates from water. As these sediments accumulate and lithify (transform into rock), they form distinct layers. Each layer tells a unique story, preserving clues about the environment, climate, and life that existed when it was deposited.
The characteristics of sedimentary rocks vary depending on their composition and origin. Conglomerates contain large, rounded pebbles, indicating high-energy environments like rivers or beaches. Sandstones, on the other hand, consist of interlocking sand grains, suggesting deposition in calmer settings. Shales, composed of compacted clay particles, often hold marine fossils, hinting at ancient seabeds. Limestones, formed from the accumulation of calcium carbonate, provide evidence of biological activity or chemical precipitation in marine environments.
The formation of sedimentary rocks is a complex process involving erosion, transportation, deposition, and lithification. Erosion breaks down rocks into smaller particles, while transportation carries these particles away by wind, water, or ice. When the particles settle out of these transport media, they form deposits. Over time, these deposits compact and cement together into solid rock through a process known as lithification.
Stratigraphy is a cornerstone of understanding Earth’s history. By studying the characteristics and sequence of rock layers, geologists can reconstruct past environments, trace the evolution of life, and interpret Earth’s tectonic history. It’s a captivating science that reveals the intricate tapestry of our planet’s past, shaping our understanding of the Earth we inhabit today.
The Geologic Timescale: Unraveling Earth’s Chronological Tapestry
In the grand narrative of Earth’s history, the geologic timescale emerges as a pivotal tool, allowing us to unravel the intricate chronology of our planet. It serves as a roadmap, guiding us through billions of years of transformative events that have shaped the world we inhabit today.
The geologic timescale is meticulously constructed based on the principle of superposition, which postulates that sedimentary rocks are deposited in chronological order, with younger layers resting atop older ones. By carefully studying the sequence of rock layers, geologists have deciphered the relative ages of different rock formations and established a comprehensive framework for measuring Earth’s history.
Each chapter in this celestial saga is further refined through a symphony of dating techniques, including radioactive decay and fossil evidence. By analyzing the decay rates of radioactive isotopes within rocks and identifying the fossilized remains of ancient organisms, scientists can determine the absolute ages of rock layers and pinpoint the timing of pivotal events in Earth’s evolution.
The geologic timescale is not merely a collection of dates; it is a dynamic narrative that paints a vivid picture of our planet’s dynamic past. It reveals epochs of volcanic eruptions, mountain-building events, and mass extinctions. It traces the evolution of life from its humble beginnings to the kaleidoscopic diversity we witness today.
Understanding the geologic timescale is akin to peering through a time-lapse camera, witnessing the intricate dance of plate tectonics, the rise and fall of sea levels, and the ceaseless sculpting of Earth’s surface by natural forces. It empowers us to comprehend the vastness of geologic time and appreciate the interconnectedness of Earth’s systems.
Dating Sedimentary Rocks Using Radioactive Decay
Unlocking the Secrets of Earth’s History
Determining the age of sedimentary rocks is crucial for unraveling the Earth’s geological past. One of the most accurate and widely used methods for this purpose is radioactive decay. This fascinating technique allows scientists to peer into the distant history of rocks and uncover the secrets they hold.
How Radioactive Decay Dates Rocks
Radioactive elements are unstable isotopes that break down over time into more stable elements. This process, known as radioactive decay, occurs at a constant rate specific to each element. By measuring the concentration of a radioactive element and its decay products in a rock, scientists can calculate how long ago the rock formed.
Direct Dating
In some cases, sedimentary rocks contain radioactive elements that allow direct dating. For example, the isotope potassium-40 (⁴⁰K) decays into argon-40 (⁴⁰Ar) over time. By measuring the ratio of ⁴⁰K to ⁴⁰Ar, scientists can directly determine the age of the rock.
Indirect Dating
Not all sedimentary rocks contain radioactive elements suitable for direct dating. However, they may contain fossils or minerals that do. Fossils of extinct organisms, known as index fossils, are found within specific rock layers and can provide relative age estimates.
Additionally, some minerals like zircon contain radioactive elements like uranium-238 (²³⁸U), which decays into lead-206 (²⁰⁶Pb) over time. By analyzing the ratio of ²³⁸U to ²⁰⁶Pb in zircons found within sedimentary rocks, scientists can indirectly determine the age of the enclosing rock.
Cross-Cutting Relationships
Other geological features can also aid in dating sedimentary rocks. For example, cross-cutting relationships occur when a younger rock formation cuts through an older rock formation. By identifying the sequence of events, scientists can determine the relative ages of the rocks involved.
Unconformities
Unconformities are breaks or gaps in the geological record where rock layers have been removed by erosion. Unconformities indicate significant periods of time when rock formation was interrupted, providing valuable information about the geological processes that have shaped the Earth.
The Geological Timescale
The knowledge gained from dating sedimentary rocks has enabled scientists to construct the geological timescale. This timeline divides Earth’s history into eons, eras, and periods, providing a framework for understanding the progression of life and geological events throughout time.
Radioactive decay is a powerful tool that unlocks the secrets of Earth’s history. By studying the decay of radioactive elements in sedimentary rocks and other geological formations, scientists can unravel the age and sequence of geological events that have shaped our planet. This knowledge forms the foundation for understanding the past, present, and future of our dynamic Earth.