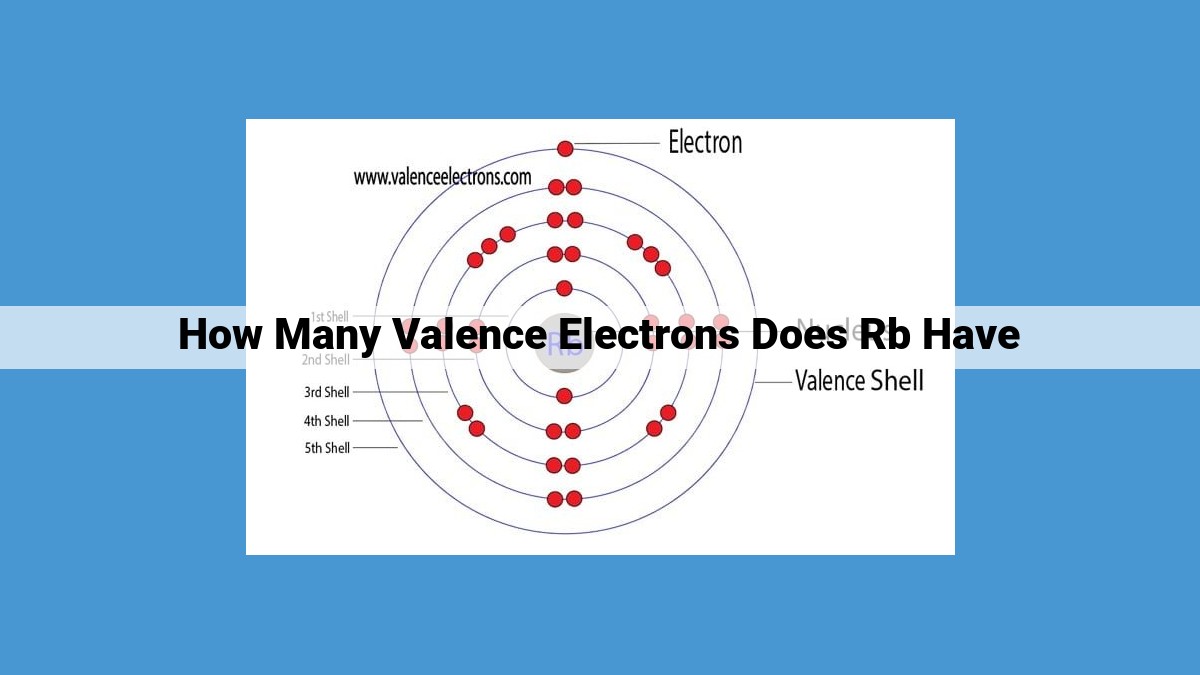
Rubidium (Rb), an alkali metal in Group 1 of the periodic table, has one valence electron in its outermost energy level (5s¹). This single valence electron plays a crucial role in determining Rb’s chemical behavior. Rb’s strong tendency to form ionic bonds with nonmetals, high reactivity, and positive ion formation potential are all attributable to its lone valence electron. This valence electron’s behavior provides insights into Rb’s chemical reactions and unique properties.
The Enigmatic World of Valence Electrons: Unraveling Rubidium’s Chemical Secrets
In the vast tapestry of chemistry, valence electrons hold a pivotal role. They’re like the master puppeteers, orchestrating an element’s chemical behavior. Meet rubidium (Rb), an alluring element with a singular valence electron. Its electronic configuration, [Kr] 5s¹, tells a compelling tale of chemical reactivity and bonding preferences.
Delving into the story of valence electrons, we encounter their innate power to determine an element’s chemistry. They’re the outermost electrons, poised at the fringes of an atom, eager to engage in chemical interactions. Valence electrons drive an element’s bonding behavior, dictating how it interacts with other atoms.
Rubidium, our protagonist, flaunts its unique valence shell configuration. Picture an atom with an inner sanctum of electrons, represented by [Kr]. This electronic fortress shields the single valence electron that resides in the 5s orbital. This lone electron, like a solitary wanderer, yearns for companionship and reactivity.
Unveiling Rubidium’s Chemical Persona
The periodic table, a map of the elements, organizes these chemical wonders based on their atomic number. Rubidium resides in Group 1, the alkali metals, a distinguished family known for their unparalleled reactivity. It also occupies Period 5, a testament to its five energy levels.
Periodicity, the periodic arrangement of elements, reveals an intriguing pattern in valence electrons. As you traverse the periodic table, the number of valence electrons corresponds to the group number. This harmonious relationship underscores the profound influence of valence electrons on an element’s chemical nature.
Oxidation number, a concept crucial to understanding chemical reactivity, measures an atom’s perceived charge. In Rb’s case, its common oxidation state is +1, indicating the loss of its solitary valence electron. This tendency to shed its valence electron makes Rb highly reactive and prompts it to form positively charged ions.
Periodicity and Valence Electrons: Unraveling the Secrets of Rubidium’s Chemistry
In the realm of chemistry, the periodic table serves as a guidebook, revealing the secrets of elements and their behaviors. Each element occupies a specific position based on its atomic number, which defines the number of protons in its nucleus.
Among the elements, rubidium (Rb) finds its home in Group 1, alongside other alkali metals. This group is nestled on the far left side of the periodic table, marking the beginning of each period or row. Periods are characterized by the number of energy levels or shells that surround the nucleus. Rb resides in Period 5, indicating that its electrons occupy five energy levels.
The interplay between an element’s position on the periodic table and its valence electrons is a fascinating dance of chemistry. Valence electrons are the electrons that reside in the outermost energy level of an atom, and they play a pivotal role in determining an element’s chemical reactivity.
Imagine Rb’s electron configuration: [Kr] 5s¹. The symbol [Kr] represents the noble gas krypton, indicating that Rb’s inner four energy levels are filled and stable. The 5s¹ notation reveals that Rb has one valence electron in its outermost 5s orbital.
This lone valence electron is the key to understanding Rb’s chemistry. It is this electron that Rb is eager to interact with other elements, seeking stability by either gaining or losing it. Rb readily loses its valence electron, achieving a stable +1 oxidation state, characteristic of alkali metals. This electron loss explains Rb’s tendency to form ionic bonds with nonmetals, resulting in positively charged rubidium ions (Rb+) and negatively charged anions.
The connection between periodicity and valence electrons is evident in Rb’s behavior. The position of Rb in Group 1 indicates that it has one valence electron, which manifests in its chemical reactivity and bonding preferences. This understanding allows us to predict the properties and behaviors of other elements based on their positions on the periodic table, unraveling the tapestry of chemical interactions and reactions.
Oxidation Number: Unlocking the Charge of Rubidium
In the realm of chemistry, understanding the behavior of elements is paramount. Oxidation number, a crucial concept in this endeavor, reveals the charge an atom possesses when involved in chemical reactions. Delving into the world of rubidium (Rb), we uncover its common oxidation state of +1, a pivotal characteristic that governs its chemical personality.
Rb’s valence electron, the electron residing in its outermost energy level, holds the key to its oxidation state. With only one valence electron, Rb has a strong tendency to lose it, resulting in a positive charge of +1. This eagerness to shed its valence electron stems from Rb’s desire to achieve a more stable, noble gas configuration.
Oxidation number profoundly influences Rb’s chemical interactions. In ionic bonding, where electrons are transferred between atoms, Rb readily forms positive ions with a +1 charge. This ionic nature enables Rb to forge strong bonds with nonmetals, such as chlorine and oxygen, leading to the formation of ionic compounds like RbCl and Rb₂O.
Furthermore, oxidation number plays a pivotal role in redox reactions, where electrons are exchanged between reactants. Rb’s ability to lose an electron makes it a reducing agent, capable of transferring electrons to other atoms or molecules. This property underlies Rb’s reactivity and its involvement in various chemical processes.
By unraveling the concept of oxidation number, we gain invaluable insights into the charge and chemical behavior of rubidium. This understanding empowers us to decipher Rb’s propensity to form ionic bonds, participate in redox reactions, and interact with other elements, paving the way for further exploration into the fascinating world of chemistry.
Chemical Bonding: Rb’s Tendency to Form Ionic Bonds
Understanding chemical bonding is crucial to unraveling the fascinating world of chemistry. Chemical bonds are the forces that hold atoms together to form molecules and compounds. Among the various types of chemical bonds, ionic bonds stand out as the electrostatic attractions between oppositely charged ions.
Rubidium (Rb), an alkali metal, exhibits a remarkable tendency to form ionic bonds, especially with nonmetals. This predilection stems from its valence electrons. Valence electrons are the electrons in an atom’s outermost energy level, and they play a pivotal role in determining an element’s chemical behavior.
Rb possesses one valence electron, which it readily loses to achieve a stable electron configuration. When Rb loses this valence electron, it transforms into a positively charged ion, known as Rb⁺. Nonmetals, on the other hand, have a high electronegativity, meaning they have a strong attraction for electrons. This disparity in electronegativity between Rb and nonmetals drives the formation of ionic bonds.
In an ionic bond, Rb⁺ donates its valence electron to a nonmetal atom, creating two oppositely charged ions. The electrostatic attraction between these ions holds the compound together. For instance, when Rb reacts with chlorine (Cl), it forms the ionic compound RbCl. In RbCl, Rb⁺ transfers its valence electron to Cl, resulting in the formation of Cl⁻ ions. The electrostatic attraction between Rb⁺ and Cl⁻ ions keeps the RbCl crystal lattice intact.
Rb’s tendency to form ionic bonds has profound implications for its chemical reactivity. Ionic bonds are generally strong and stable, making Rb compounds highly soluble in water. Rb also reacts vigorously with other elements, particularly nonmetals, to form a wide range of ionic compounds. These compounds play crucial roles in various applications, including batteries, fertilizers, and glass manufacturing.
Unveiling the Number of Valence Electrons in Rb
- Recapitulate key concepts to answer the central question.
- State that Rb possesses one valence electron in its outermost energy level (5s¹).
- Emphasize the high reactivity and positive ion formation potential of Rb due to its single valence electron.
Unveiling the Valence Electron Count of Rubidium
In the intriguing realm of chemistry, valence electrons play a pivotal role in shaping the behavior of elements. They determine chemical interactions and dictate the formation of bonds that hold molecules together. Let’s embark on a journey to understand the significance of valence electrons, taking rubidium (Rb) as our guide.
Rb, a member of the alkali metal family, holds a special place in the Periodic Table. Its unique position in Group 1 (alkali metals) and Period 5 reveals essential clues about its valence electrons. According to the Periodic Table’s organization based on atomic number, Rb has 37 protons and 37 electrons.
The valence shell, or outermost energy level, of Rb holds the key to understanding its chemical behavior. Rb’s valence shell configuration is [Kr] 5s¹, indicating that it possesses one valence electron in the 5s orbital. This lone valence electron is the driving force behind Rb’s chemical reactivity.
Due to its single valence electron, Rb has a strong tendency to lose this electron and form positive ions. This electron loss results in an oxidation state of +1, a common characteristic of alkali metals. Rb’s high reactivity and positive ion formation potential make it an excellent candidate for forming ionic bonds with nonmetals.
In conclusion, Rb’s single valence electron, residing in its outermost 5s orbital, dictates its chemical behavior. This lone electron drives Rb’s strong reactivity, positive ion formation potential, and preference for ionic bonding. Understanding the significance of valence electrons provides a deeper understanding of Rb’s unique properties and its role in chemical reactions.