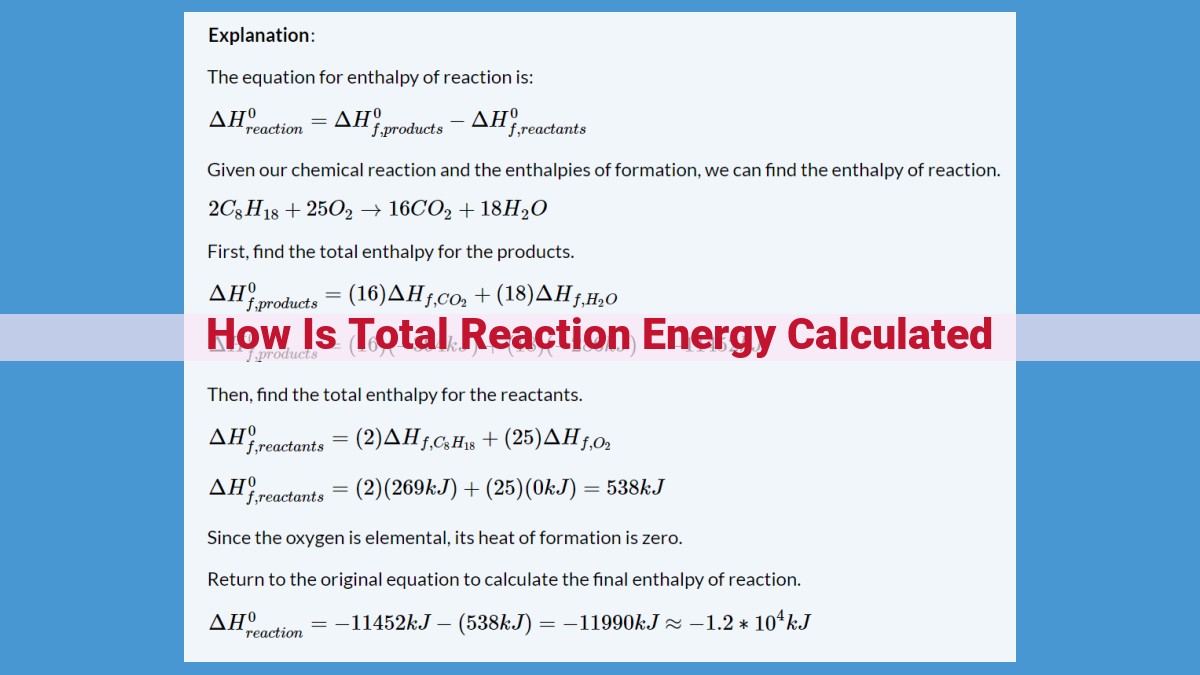
Total reaction energy can be calculated using the enthalpy change, which is the difference in enthalpy between the reactants and products. Enthalpy change can be determined using the standard enthalpy of formation, a reference point for enthalpy calculations. Hess’s law allows for the prediction of enthalpy changes for complex reactions by combining reaction energies. Bond enthalpy, the energy required to break chemical bonds, contributes to the overall reaction energy. Resonance, including aromaticity, can impact bond strengths and energies, affecting reaction outcomes.
Dive into the Energetic World of Enthalpy Change
Enthalpy, the measure of energy within a system, undergoes captivating transformations in countless chemical reactions. Enthalpy change, denoted as ΔH, is the difference in enthalpy between the initial and final states of a reaction. This change tells us whether heat is released or absorbed.
Nestled within this energetic realm, the standard enthalpy of formation serves as a pivotal reference point. It’s the enthalpy change associated with the formation of one mole of a compound from its constituent elements in their standard states. This key concept enables us to calculate enthalpy changes via Hess’s Law.
Hess’s Law unveils the interconnectedness of reactions. It states that the enthalpy change of a reaction is equal to the sum of the enthalpy changes of the individual steps required to reach the same final state. By carefully dissecting complex reactions into simpler components, we can harness the power of Hess’s Law to unravel their energetic consequences.
Standard Enthalpy of Formation: The Reference Point
- Introduce the concept of standard enthalpy of formation as the reference point for enthalpy calculations.
- Describe how it is determined through experimental measurements.
- Explore its relationship to elemental enthalpies and bond enthalpy.
Standard Enthalpy of Formation: The Cornerstone of Enthalpy Calculations
In the realm of thermodynamics, enthalpy change plays a pivotal role in understanding the energy flow within chemical reactions. To quantify this energy exchange, scientists have devised a reference point known as the standard enthalpy of formation.
Imagine a chemist mixing reactants to create a new compound. As the reaction progresses, energy may be absorbed or released. The standard enthalpy of formation provides the baseline against which this energy change is measured. It represents the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states.
The standard state of an element is the most stable form at 25°C and 1 atmosphere of pressure. For example, the standard state of carbon is solid graphite, while for hydrogen it is diatomic gas (H2).
Measuring Standard Enthalpy of Formation:
Determining the standard enthalpy of formation involves meticulous experimental measurements. Chemists burn compounds in a calorimeter to measure the released heat, known as the heat of combustion. This data, combined with the enthalpies of formation of the reactants, allows scientists to calculate the standard enthalpy of formation of the product.
Relationship to Elemental Enthalpies and Bond Enthalpy:
The standard enthalpy of formation is intricately linked to the enthalpies of the constituent elements and the bond enthalpies within the compound. The enthalpy of formation of an element in its standard state is zero by definition. The enthalpy change associated with breaking a bond is known as the bond enthalpy.
For example, the standard enthalpy of formation of water (H2O) is -285.8 kJ/mol. This negative value indicates that energy is released when hydrogen and oxygen react to form water. This energy release is attributed to the formation of strong O-H bonds, which have a high bond enthalpy.
By understanding the standard enthalpy of formation, chemists gain a valuable reference point for analyzing and predicting the energy changes in chemical reactions. It serves as a cornerstone in the study of thermodynamics and aids in unraveling the intricate energetics of countless chemical processes.
Hess’s Law: Unlocking Enthalpy Changes in Complex Reactions
In the realm of thermodynamics, Hess’s law emerges as a mathematical powerhouse, guiding us through the intricate pathways of enthalpy changes in complex reactions.
The Essence of Hess’s Law
Imagine you have a complex reaction, like building a house. Breaking it down into smaller steps, like laying the foundation and constructing the walls, makes it easier to calculate the total energy required. Hess’s law operates on a similar principle. It allows us to predict the enthalpy change of an overall reaction by combining the enthalpy changes of individual steps, just like adding up the energy needed for each step in building the house.
The Standard Reference Point
To make this magic happen, Hess’s law relies on a crucial reference point: the standard enthalpy of formation (∆Hf°). This value tells us the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states. It’s like the energy blueprint for building a compound from scratch.
Armed with ∆Hf° values and Hess’s law, we can calculate the enthalpy change of any reaction, no matter how complex. We simply add up the ∆Hf° values of the products and subtract the ∆Hf° values of the reactants. The resulting value is the overall enthalpy change (∆H), which tells us whether the reaction is exothermic (releases energy) or endothermic (absorbs energy).
Harnessing Hess’s Power
Hess’s law empowers us to unravel the mysteries of complex reactions in countless scenarios. For instance, we can:
- Predict the enthalpy change of a reaction without the need for direct experimentation.
- Determine the heat released or absorbed during a chemical process.
- Optimize reaction conditions to maximize energy efficiency.
- Design new chemical reactions with desired outcomes.
Hess’s law is a cornerstone of thermodynamics, providing a mathematical framework for understanding and predicting enthalpy changes in complex reactions. By breaking down these reactions into smaller steps and using the standard enthalpy of formation as a reference, Hess’s law has revolutionized our ability to harness the power of chemical reactions in a wide range of applications.
Bond Enthalpy: The Energy of Breaking and Forming Chemical Bonds
Bond enthalpy is a fundamental concept in chemistry that quantifies the energy involved in breaking or forming chemical bonds. It plays a crucial role in determining the stability of molecules and the overall energy changes that occur during chemical reactions. Understanding bond enthalpy is essential for comprehending the behavior of chemical systems.
Defining Bond Enthalpy
Bond enthalpy is defined as the enthalpy difference between the reactants and products when a single bond is broken. In other words, it represents the energy required to break a specific bond in a molecule. For example, the bond enthalpy of the C-H bond in methane is approximately 436 kJ/mol, meaning that it takes 436 kJ of energy to break this bond.
Estimating Bond Enthalpy
Bond enthalpy can be estimated using experimental data or theoretical methods. Experimental techniques involve measuring the enthalpy change of a reaction that specifically breaks a particular bond. Theoretical methods rely on quantum mechanical calculations to predict bond enthalpies based on molecular structure.
Contributions to Standard Enthalpy of Formation
Bond enthalpy is a key contributor to the standard enthalpy of formation of a compound. The standard enthalpy of formation is the enthalpy change when 1 mole of a compound is formed from its constituent elements in their standard states. Each bond in the compound contributes its bond enthalpy to the overall standard enthalpy of formation.
Influence on Reaction Energies
Bond enthalpy also affects the overall energy changes that occur during chemical reactions. When bonds are broken, energy is consumed, while when bonds are formed, energy is released. The balance between bond breaking and bond formation determines the enthalpy change of a reaction.
By understanding bond enthalpy, chemists can predict the stability of molecules, estimate the energy required for chemical reactions, and design synthetic pathways to achieve desired transformations.
Resonance: Delocalized Electrons and Molecular Stability
In the realm of chemistry, the concept of resonance plays a pivotal role in understanding how molecules behave and interact. Resonance arises when a molecule possesses multiple Lewis structures with equivalent energy distributions. In these structures, electrons are delocalized, meaning they are not confined to a specific atom or bond but, rather, are spread out over several atoms. This delocalization of electrons results in a more stable molecule.
The stabilization effect of resonance stems from the fact that it lowers the overall energy of the molecule. When electrons are spread out over multiple atoms, the molecule becomes less reactive and more resistant to chemical change. This is because the delocalized electrons are less likely to participate in chemical reactions, as they are not localized in one specific location.
One of the most striking examples of resonance is the benzene molecule. Benzene is a cyclic molecule composed of six carbon atoms and six hydrogen atoms, arranged in a hexagonal shape. The unique stability of benzene can be attributed to resonance. In benzene, the six carbon atoms form a ring with alternating single and double bonds. However, the electrons in the double bonds are not confined to specific carbon-carbon bonds; rather, they are delocalized around the entire ring. This delocalization results in the formation of a particularly stable aromatic ring system.
The concept of resonance not only explains the stability of molecules but also has implications for their reactivity. Molecules with delocalized electrons are less likely to react with other molecules, as the delocalized electrons are less available for bonding. This can affect the molecule’s chemical properties and reactivity patterns.
Understanding resonance is crucial for comprehending the behavior of molecules and predicting their reactivity. By considering the delocalization of electrons, chemists can gain insights into the stability and properties of various chemical compounds.
Aromaticity: A Special Case of Resonance
- Define aromaticity as cyclic, planar molecules with delocalized electrons.
- Explain the special stability of aromatic molecules due to resonance and other factors.
- Highlight how resonance underlies aromaticity and its unique properties.
Aromaticity: The Symphony of Delocalized Electrons
In the realm of chemistry, aromaticity takes center stage as a unique phenomenon that captivates scientists and students alike. Aromatic molecules are distinguished by their remarkable cyclic, planar structure and the presence of delocalized electrons.
Imagine a dance of electrons, gracefully moving within a molecule’s framework. In aromatic molecules, these electrons are not confined to specific bonds but spread out across the entire ring. This delocalization gives rise to extra stability, making aromatic molecules less reactive and more resistant to chemical changes.
The story of resonance unfolds as the key player behind aromaticity. Resonance allows electrons to resonate between different molecular structures, creating hybrid structures that contribute to the molecule’s stability. In aromatic molecules, resonance achieves a harmonious balance, distributing the electrons evenly across the ring.
One of nature’s most iconic examples of aromaticity is benzene. This six-membered ring of carbon atoms possesses a planar structure with three alternating double bonds. Despite its appearance, benzene does not undergo the typical chemical reactions of alkenes due to its aromatic character. The delocalized electrons in benzene create a ring of stability that sets it apart from its non-aromatic counterparts.
The unique properties of aromatic molecules extend beyond their exceptional stability. Their electronic structure also influences their chemical behavior. Aromatic compounds are often more reactive towards certain types of reactions, such as electrophilic aromatic substitution. This reactivity stems from the rich electron density in the aromatic ring, which makes it a target for certain chemical species.
In conclusion, aromaticity is a mesmerizing dance of electrons, conferring unique stability and chemical properties to molecules. It underpins the exceptional characteristics of aromatic compounds, making them essential in many areas of science and industry.