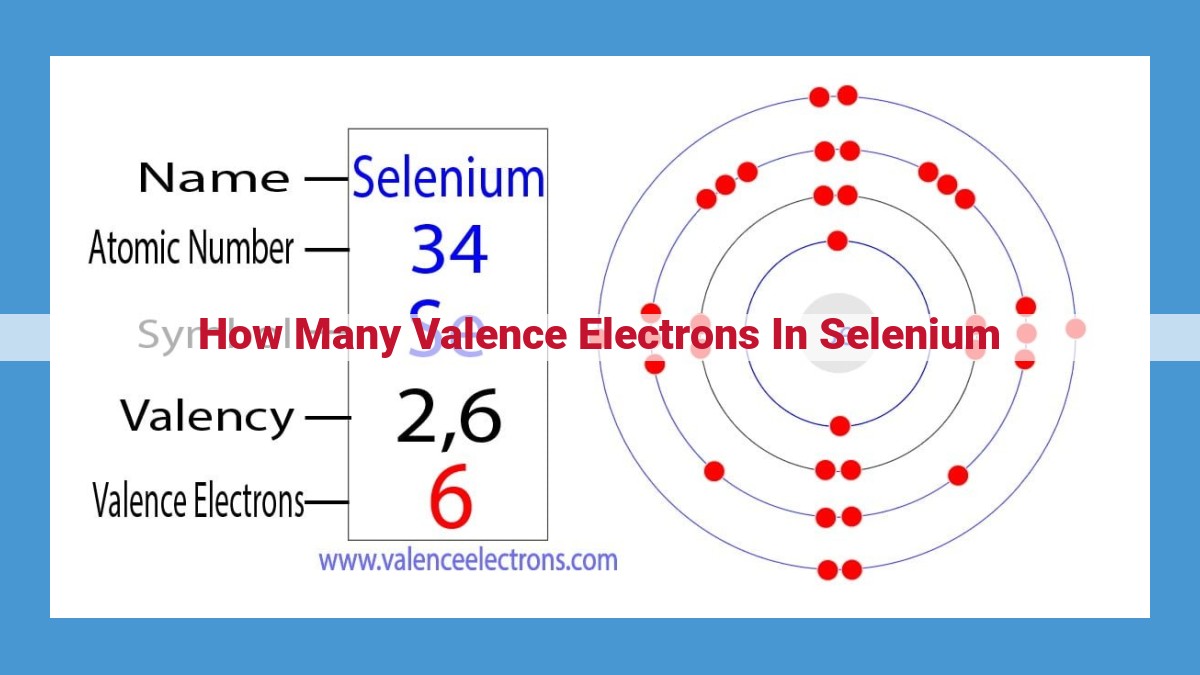
Selenium, a versatile chalcogen in Group 16 of the periodic table, possesses six valence electrons due to its electron configuration of [Ar] 3d¹º 4s² 4p⁴. This configuration indicates that selenium has four valence electrons in the outermost 4p orbital, making it highly reactive and capable of forming diverse chemical bonds.
Valence Electrons: A Gateway to Bonding
- Explain electron configuration and how it determines valence electrons.
- Discuss electronegativity and its role in predicting bonding behavior.
- Describe the types of chemical bonds (covalent, ionic, etc.) facilitated by valence electrons.
Valence Electrons: The Key to Bonding
Imagine a dance floor teeming with electrons, the tiny particles that whirl around the nucleus of an atom. Among these electrons, a special group takes center stage: valence electrons. These outermost electrons determine an atom’s bonding behavior, acting like social butterflies that interact with other atoms to form chemical bonds.
Electron Configuration and Valence Electrons
Each element’s atomic number reveals the number of protons and electrons it possesses. The distribution of electrons in energy levels or orbitals is known as electron configuration. The electrons in the outermost energy level are the valence electrons.
Electronegativity
Electronegativity measures an atom’s ability to attract electrons towards itself. This property plays a crucial role in predicting bonding behavior. Highly electronegative atoms, like fluorine, have a strong grip on their valence electrons, while less electronegative atoms, like sodium, are willing to let go of their valence electrons.
Types of Chemical Bonds
Valence electrons facilitate a dance of attraction and repulsion, forming various types of chemical bonds:
- Covalent Bonds: When two atoms share valence electrons to form a strong, covalent bond, like the one between hydrogen and chlorine in hydrochloric acid.
- Ionic Bonds: Highly electronegative atoms steal valence electrons from less electronegative atoms, creating an ionic bond, as in the bond between sodium and chlorine in table salt.
- Metallic Bonds: In metals, valence electrons freely move like a sea, creating strong metallic bonds that give metals their characteristic luster and malleability.
Selenium: A Versatile and Enigmatic Chalcogen
Chalcogens: A Unique Family of Elements
Nestled within the periodic table’s Group 16, chalcogens are a fascinating group of elements that share a common characteristic: six valence electrons. Selenium, with its atomic number 34, takes its rightful place among this enigmatic family, alongside oxygen, sulfur, tellurium, and polonium.
Periodic Trends Shaping Selenium’s Properties
The periodic table serves as an invaluable guide to understanding the properties of elements. As we move down Group 16, we encounter a gradual increase in atomic radius, electronegativity, and metallic character. This trend reflects the increasing number of energy levels and decreasing electrostatic attraction between the nucleus and outermost electrons.
In the case of selenium, its intermediate position in Group 16 grants it a balanced set of properties. It exhibits a moderate atomic radius, moderate electronegativity, and modest metallic character. These characteristics contribute to its versatility and wide-ranging applications.
Toxicity: A Double-Edged Sword
While selenium’s versatility makes it a valuable resource, it also poses a potential health risk. High levels of selenium can induce toxicity, causing a variety of symptoms including hair loss, nausea, and fatigue. Paradoxically, low levels of selenium can lead to deficiencies, resulting in conditions such as Keshan disease.
To navigate this delicate balance, it is crucial to maintain optimal selenium levels. The recommended daily intake for adults ranges from 55 to 200 micrograms. While selenium is naturally found in foods like fish, eggs, and nuts, supplementation may be necessary in certain circumstances.
Selenium, a versatile chalcogen, embodies the intricate dance between properties and health implications. Its position in Group 16 shapes its unique characteristics, making it a valuable resource in various fields. However, understanding its potential toxicity is paramount to harnessing its benefits safely and achieving optimal health.
The Periodic Table: A Chemical Atlas
Unveiling the Blueprint of Matter
In the grand tapestry of chemistry, the periodic table serves as a cosmic map, guiding us through the vast expanse of elements that make up our universe. It’s a masterpiece of organization, where elements are arranged in a systematic manner that reveals their intricate relationships and properties.
Vertical Pathways: The Groups
Along the vertical columns, we encounter groups of elements that share a common electronic configuration. This cosmic kinship manifests in their valence electrons, the outermost electrons that determine an element’s bonding behavior. Group numbers precisely indicate the number of valence electrons, providing invaluable insights into their chemical prowess.
Horizontal Layers: The Periods
Horizontally, the periodic table is divided into periods, representing the number of electron shells within the elements. As we traverse from left to right across a period, the atomic number increases, and the elements gain protons and neutrons. This progressive change in atomic structure influences their size, electronegativity, and reactivity, creating a fascinating interplay of properties.
Noble Sanctuary: The Full Valence Shell
At the far right of the periodic table reside the noble gases, elements that have achieved a state of chemical tranquility. Their valence shells are brimming with a full complement of electrons, granting them a rare stability that makes them unyielding to chemical bonds. They are the enigmatic spectators of the chemical world, indifferent to the bonding drama unfolding around them.
A Chemical Treasure Map
The periodic table is not merely a static arrangement of elements. It’s a dynamic tool that empowers chemists to predict and understand the behavior of matter. By deciphering the secrets encoded within its structure, we unravel the complexities of chemical reactions, unravel the mysteries of molecular interactions, and unlock the potential for scientific advancements that shape our world.
Atomic Number: The Identity and Composition of Elements
In the realm of chemistry, elements are the fundamental building blocks of the universe. Each element is unique, possessing its own set of properties that define its character and behavior. The atomic number stands as the defining characteristic that distinguishes one element from another.
Delving into the atomic structure, we encounter the trio of subatomic particles: protons, neutrons, and electrons. Protons, residing in the nucleus, carry a positive charge that determines the element’s atomic number. Neutrons, also in the nucleus, possess no charge, while electrons, orbiting the nucleus, carry a negative charge.
The atomic number of an element is equal to the number of protons in its nucleus. This fundamental value serves as the element’s fingerprint, providing its unique identity. It plays a crucial role in determining the element’s position on the periodic table, a roadmap that organizes elements based on their properties and structure.
Furthermore, the atomic number has profound implications for the element’s chemical behavior. The valence electrons, the electrons in the outermost shell of an atom, govern the element’s ability to form chemical bonds. The number of valence electrons corresponds to the group number on the periodic table, providing valuable insights into the element’s reactivity and bonding preferences.
The concept of isotopes adds another layer of complexity to the atomic landscape. Isotopes are variations of an element that share the same atomic number but differ in the number of neutrons. This variation in neutron count affects the mass of the atom without altering its chemical properties. Understanding isotopes is essential for applications such as nuclear chemistry and mass spectrometry.
In summary, the atomic number serves as the defining characteristic of an element, providing its identity and dictating its chemical behavior. By understanding the interplay between protons, neutrons, electrons, and isotopes, we gain a deeper appreciation for the fundamental nature of matter.
Group Number: Decoding Valence Electrons and Shaping Chemical Behavior
In the riddle of the Periodic Table, the group number of an element holds a crucial key to unlocking its chemical secrets. It’s a clue to the number of valence electrons, the outermost electrons orbiting the nucleus, which play a pivotal role in shaping the element’s chemical personality and behavior.
Unveiling the Group Number’s Significance
The group number is the vertical column in which an element resides on the Periodic Table. It corresponds to the number of valence electrons the element possesses. For instance, Group 1 elements have one valence electron, denoted as 1s¹. Group 2 elements have two valence electrons (2s²), while Group 18 elements, known as noble gases, boast a full set of eight valence electrons (ns² np⁶), making them chemically inert.
Valence Electrons: Architects of Chemical Properties
Valence electrons are the movers and shakers in the chemical world, dictating an element’s ability to form bonds with other atoms. Elements with similar valence electron configurations exhibit similar chemical properties, a trend that becomes apparent as we traverse the horizontal rows of the periodic table, called periods.
Periodic Trends: Mapping Chemical Behavior
As we move from left to right across a period, the number of valence electrons increases, leading to a gradual increase in electronegativity. Electronegativity measures an element’s ability to attract electrons toward its nucleus, influencing its bonding behavior.
For instance, in Period 2, fluorine (Group 17) is highly electronegative due to its seven valence electrons, making it a ** voracious electron acceptor**. Conversely, sodium (Group 1) has only one valence electron, making it highly electropositive and eager to donate its electron, forming ionic bonds with electronegative elements.
The group number on the Periodic Table is a treasure map to an element’s valence electrons, the gatekeepers to its chemical behavior. By deciphering the number of valence electrons, we can predict bonding tendencies, electronegativity, and periodic trends. This knowledge empowers us to understand the interplay of elements and their ability to form the vast array of compounds that shape our world.