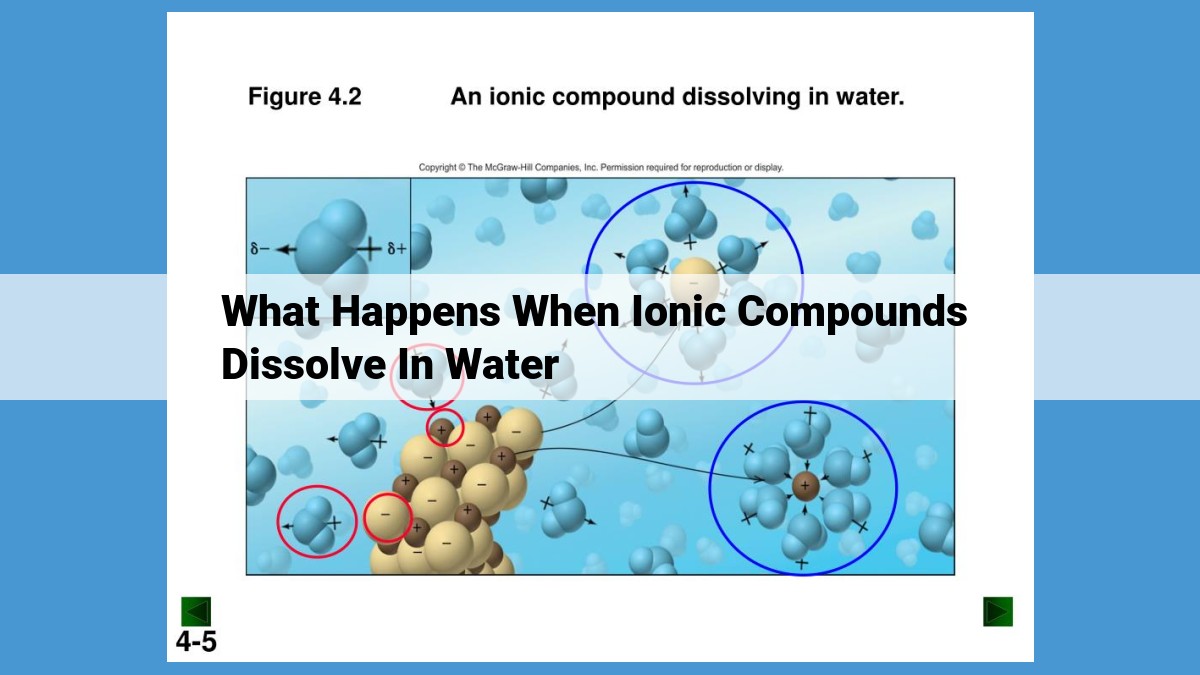
Upon dissolving in water, ionic compounds dissociate into their constituent ions due to electrostatic interactions. The formed ions become solvated by water molecules, forming hydration shells that stabilize them. The enthalpy of hydration and lattice energy, along with solvent effects, determine the enthalpy of solution and thus the solubility of the compound. Factors like temperature, pressure, and the common ion effect modulate solubility by influencing the equilibrium between hydration and lattice formation.
Formation of Ions: The Basics
Imagine atoms as little worlds, with a central nucleus surrounded by orbiting electrons. In the realm of chemistry, the dance of electrons between atoms governs how they interact and form substances.
Electrovalence: The Key to Ionic Bonds
When atoms join forces, they can do so by sharing electrons (covalent bonding) or by transferring electrons (ionic bonding). Ionic bonds form when one atom has a strong tendency to donate an electron, and another atom is eager to accept it. The ability of an atom to donate or accept electrons is determined by its electronegativity, a measure of its electron-attracting power.
Electronegativity and Ion Formation
Atoms with high electronegativity, such as fluorine and oxygen, have a strong pull on electrons, making them likely to accept electrons and become anions. In contrast, atoms with low electronegativity, like sodium and potassium, have a weak attraction for electrons and readily give them up, becoming cations.
Ionization Energy: The Quantum Leap
The energy required to remove an electron from an atom is known as its ionization energy. This energy barrier determines how easily an atom can become a cation. Atoms with low ionization energy, like the alkali metals, are more likely to form cations than those with high ionization energy, like the noble gases.
The Birth of Ions
When an atom loses or gains electrons, it becomes electrically charged. Ions are atoms that have a net positive or net negative charge. The transfer of electrons leads to the formation of oppositely charged ions, which are then attracted to each other by electrostatic forces, forming ionic compounds.
Hydration of Ions: A Stable Embrace
In the ever-dynamic world of chemistry, ions play an indispensable role in shaping the properties and behavior of substances. Among the many intriguing aspects of ion chemistry is the concept of hydration, a phenomenon that profoundly influences the stability of ions in aqueous solutions.
Electrostatic Interactions: The Driving Force
Ions, being electrically charged, possess a remarkable ability to interact with other electrically charged species through electrostatic forces. These forces act like invisible magnets, drawing ions together or repelling them apart. In the case of hydration, it is the electrostatic attraction between water molecules and ions that drives the formation of hydration shells.
Hydration Shells: A Protective Shield
Water molecules, themselves dipolar in nature, have a positively charged end and a negatively charged end. When an ion is introduced into an aqueous solution, the water molecules reorient themselves, forming a spherical shell around the ion. This hydration shell shields the ion from the surrounding water molecules, minimizing the disruptive effects of the ion’s charge and rendering it more stable.
The Significance of Hydration Shells
Hydration shells play a pivotal role in stabilizing ions in aqueous solutions. They prevent ions from reacting with other species, impede their precipitation from solution, and enhance their solubility. Moreover, hydration shells influence the transport properties of ions, affecting their diffusion and mobility in solution.
Delving Deeper into Hydration
The formation of hydration shells is a complex process governed by several factors, including the size, charge, and polarizability of the ion, as well as the temperature and solvent properties. Understanding the intricacies of hydration is crucial for comprehending the behavior of ions in aqueous environments, which are ubiquitous in nature and play a vital role in biological processes and technological applications.
Lattice Energy: The Unsung Strength of Ionic Bonds
In the fascinating realm of ionic chemistry, the formation of ionic bonds involves a captivating interplay of electrovalence, electronegativity, and ionization energy. However, the strength of this ionic embrace is not merely confined to these factors. Enter lattice energy, a fundamental property that governs the stability and behavior of ionic compounds.
Picture a lattice, a three-dimensional arrangement of ions in a crystal structure. These ions, held together by their electrostatic attraction, form a solid network. The strength of this crystalline bond is what we refer to as lattice energy.
Two crucial factors determine the magnitude of lattice energy: ionic radius and crystal structure. Smaller ions with a higher charge density generate stronger electrostatic attraction, leading to higher lattice energy. Similarly, a more symmetric and tightly packed crystal structure allows for optimal ion-ion interactions, again contributing to enhanced lattice energy.
To unravel the numerical value of lattice energy, scientists have devised the Born-Haber cycle, a valuable tool that combines thermochemical data to calculate this elusive property. This cycle involves a series of energy changes associated with the formation of an ionic compound from its gaseous ions.
In essence, lattice energy is a crucial parameter that reflects the strength of the ionic bond and governs the stability and solubility of ionic compounds. Understanding this concept sheds light on the fundamental nature of ionic interactions and their wide-ranging applications in various fields.
Enthalpy of Hydration: The Energetic Embrace of Ions and Water
Prepare yourself for a captivating tale that unravels the hidden forces behind the enthalpy of hydration, where ions and water engage in an energetic dance.
When an ion plunges into the vast ocean of water molecules, it’s like a magnet drawing in tiny water dipoles. The positive ion attracts the negative end of water molecules, while the negative ion does the reverse. This electrostatic attraction, akin to a symphony of charged particles, releases energy. This burst of energy is what we call the enthalpy of hydration.
But the story doesn’t end there. The water molecules, like loyal bodyguards, surround the ion, forming a protective shield known as a hydration shell. These shells not only stabilize the ion but also prevent it from interacting with other ions, ensuring their harmonious coexistence in solution.
The enthalpy of hydration is a crucial factor in determining the solubility of ionic compounds. The more favorable the hydration process (i.e., the more negative the enthalpy of hydration), the more soluble the ionic compound. This is because the hydration shell’s stabilizing effect makes it easier for the ions to break free from their crystal lattice and dissolve into water.
So, next time you encounter an ionic solution, remember this enchanting tale of electrostatic forces and solvation energy. It’s a story of how ions and water join forces, releasing energy and creating a stable symphony of charged particles.
Enthalpy of Solution: The Dance of Energy
When ionic solids dissolve in water, a fascinating dance of energy takes place. This energy exchange is captured in the enthalpy of solution, a measure of the heat released or absorbed during the process.
Breaking the Ionic Bond: Lattice Energy
The first step in dissolving an ionic solid is to break apart its crystalline structure. This requires overcoming the strong electrostatic forces holding the ions together. The energy required to do this is known as lattice energy. The larger the ions and the more opposite their charges, the greater the lattice energy.
Hydration: Embracing the Ions
As the ions break free from the crystal, they are surrounded by water molecules. These water molecules form a hydration shell around the ions, attracted by the opposite charges. This process releases energy due to the electrostatic interactions between the ions and the water molecules.
Enthalpy of Hydration: A Delicate Balance
The enthalpy of hydration is the energy released when ions are surrounded by water molecules. This energy is typically exothermic, meaning that heat is released. However, the extent of hydration depends on the size and charge of the ions. Smaller ions have a stronger hydration energy than larger ions, and ions with multiple charges have a more pronounced hydration effect.
Enthalpy of Solution: The Balancing Act
The enthalpy of solution is the net energy change that occurs when an ionic solid dissolves in water. It is the sum of the endothermic lattice energy and the exothermic enthalpy of hydration. If the enthalpy of hydration is greater than the lattice energy, the enthalpy of solution will be negative, indicating an exothermic process. Conversely, if the lattice energy is greater than the enthalpy of hydration, the enthalpy of solution will be positive, indicating an endothermic process.
Solvent Effects: A Supporting Cast
The enthalpy of solution is also influenced by the solvent. Water is a polar solvent, meaning it has a partial positive and negative charge. This allows it to interact strongly with ions. However, other solvents, such as alcohol or acetone, may have different polarities and can affect the enthalpy of solution.
The enthalpy of solution is a complex but crucial concept that helps us understand the energetics of ionic dissolution. It is a dance between the breaking of ionic bonds and the formation of hydration shells, a balance that determines whether a dissolution process releases or absorbs heat.
Solubility: Striking the Perfect Balance
In the realm of chemistry, solubility plays a crucial role in determining the behavior of ionic compounds. It’s the ability of a substance to dissolve in a solvent, creating a homogeneous mixture. Understanding the factors that influence solubility is essential for comprehending the chemical interactions that govern our world.
Temperature: The Heat Factor
Temperature is a significant factor affecting solubility. As temperature rises, the kinetic energy of solvent molecules increases. This increased energy helps break down the forces holding solute particles together, allowing them to dissolve more readily. Thus, solubility generally increases with temperature.
Pressure: Making Space for Solubility
In the case of gases dissolved in liquids, pressure also plays a role. According to Henry’s law, the solubility of a gas is directly proportional to its partial pressure above the liquid. This means that increasing the pressure enhances gas solubility in liquids.
Solute-Solvent Interactions: A Chemical Dance
The nature of the solute-solvent interactions strongly influences solubility. Polar solvents, like water, interact favorably with polar or ionic solutes. This attraction enhances the ability of the solute to dissolve. Nonpolar solvents, on the other hand, are less effective at solvating polar solutes, resulting in lower solubility.
Common Ion Effect: Le Chatelier’s Balancing Act
The presence of a common ion in solution can significantly reduce the solubility of a compound. According to Le Chatelier’s principle, the equilibrium shifts towards reducing the concentration of the common ion. In the case of solubility, the addition of a common ion drives the equilibrium towards the formation of solid precipitate, resulting in decreased solubility.
Factors Influencing Solubility: Temperature, Pressure, and pH
Temperature:
Temperature plays a crucial role in determining the solubility of ionic compounds. Generally, as temperature increases, the solubility of ionic compounds increases. This is because higher temperatures provide more kinetic energy to the solvent molecules, allowing them to more effectively break apart the ion-dipole interactions that hold the ions in the solid lattice. In other words, the higher the temperature, the more energetic the solvent molecules become, and the easier it is for them to dissolve the ionic compound.
Pressure:
Pressure has a relatively small effect on the solubility of ionic compounds. When pressure is applied to a solution, the solvent molecules are forced closer together, making it more difficult for them to solvate the ions. This results in a decrease in solubility. However, the effect of pressure on solubility is generally only significant for gases, as they are much more compressible than liquids or solids.
pH:
pH can also affect the solubility of ionic compounds, particularly those that contain weak acids or bases. For example, the solubility of calcium carbonate (CaCO₃) decreases as the pH decreases. This is because at low pH, the concentration of hydrogen ions is high, which causes the formation of insoluble calcium hydrogen carbonate ((Ca(HCO₃)₂)].
In general, the factors that influence solubility are complex and interconnected. However, by understanding the basic principles involved, it is possible to predict how solubility will change under different conditions. This knowledge is essential for a wide range of applications, from the design of industrial processes to the development of new drugs.
Common Ion Effect: Le Chatelier’s Principle in Action
In the realm of chemistry, ions play a vital role in determining the solubility of ionic compounds. The common ion effect is a phenomenon that illustrates how the addition of a common ion can influence the solubility of an ionic compound. To delve into this concept, we must first understand Le Chatelier’s principle.
Le Chatelier’s principle states that when a change is applied to a system in equilibrium, the system will shift in a direction that counteracts the change. In the case of solubility equilibrium, the addition of a common ion shifts the equilibrium towards the solid phase, decreasing the solubility of the ionic compound.
How does it work?
Consider a solution containing a saturated ionic compound, MX. The equilibrium equation for the dissolution of MX is:
MX(s) <=> M+(aq) + X-(aq)
If we add a common ion, such as M+ or X-, to the solution, the equilibrium will shift to the left to counteract the increase in common ion concentration. This shift decreases the solubility of MX by reducing the concentration of M+ and X- ions in solution.
Think of it as a tug-of-war between the ions. When you add more ions on one side, the system pulls back by forming more solid MX to balance the equation, thus decreasing the number of ions in solution.
Applications of the Common Ion Effect
The common ion effect has practical applications in various fields:
- Desalination: By adding sodium chloride to seawater, the solubility of calcium carbonate is decreased, preventing scale formation in desalination plants.
- Pharmaceuticals: The solubility of certain drugs can be controlled by adjusting the concentration of common ions in the solution.
- Environmental chemistry: The common ion effect can be used to control the solubility of metals in contaminated water or soil.
Understanding the common ion effect is essential for comprehending the behavior of ions in solution and predicting the solubility of ionic compounds. By applying Le Chatelier’s principle, chemists can manipulate solubility to achieve desired outcomes in various fields.
Applications of Ionic Solutions: Beyond the Classroom
In the realm of chemistry, ionic solutions extend their significance far beyond the confines of textbooks and experiments. These charged compounds play a pivotal role in numerous real-world applications, impacting our daily lives in myriad ways.
Electroplating: Precision Coating
Electroplating harnesses the power of ionic solutions to coat surfaces with a thin layer of metal. By passing an electric current through a solution containing metallic ions, these ions deposit onto the desired surface, forming a durable and protective coating. Electroplating finds widespread use in industries such as electronics, jewelry, and automotive to enhance corrosion resistance and improve appearance.
Batteries: Storing Electrical Energy
Ionic solutions form the heart of batteries, devices that store electrical energy. Inside a battery, two electrodes are immersed in a solution containing ions. When a circuit is connected, chemical reactions occur within the solution, releasing electrons that can then flow and power our electronic devices. Lithium-ion batteries, commonly found in laptops and smartphones, rely on the movement of lithium ions between the electrodes.
Fertilizers: Nourishing the Soil
Ionic solutions are essential for plant growth and agriculture. Fertilizers contain a variety of ions, such as nitrogen, phosphorus, and potassium, which are absorbed by plants through their roots. These ions provide the nutrients necessary for photosynthesis, growth, and seed production, ensuring bountiful harvests that feed the global population.
Pharmaceuticals: Healing Properties
Ionic solutions have a significant presence in the pharmaceutical industry. Many medications, such as antibiotics and painkillers, exist as ionic compounds dissolved in water. These solutions are easily absorbed by the body, allowing them to effectively target specific ailments. Additionally, intravenous fluids, which often contain electrolytes like sodium and chloride ions, are commonly used in hospitals to restore hydration and maintain fluid balance in patients.
In conclusion, ionic solutions are versatile and indispensable tools that extend their influence beyond theoretical concepts. From enhancing surfaces with electroplating to powering our devices through batteries, from nourishing crops with fertilizers to providing therapeutic treatments in pharmaceuticals, ionic solutions play a crucial role in shaping our world. Their applications continue to expand, promising even greater advancements in the years to come.