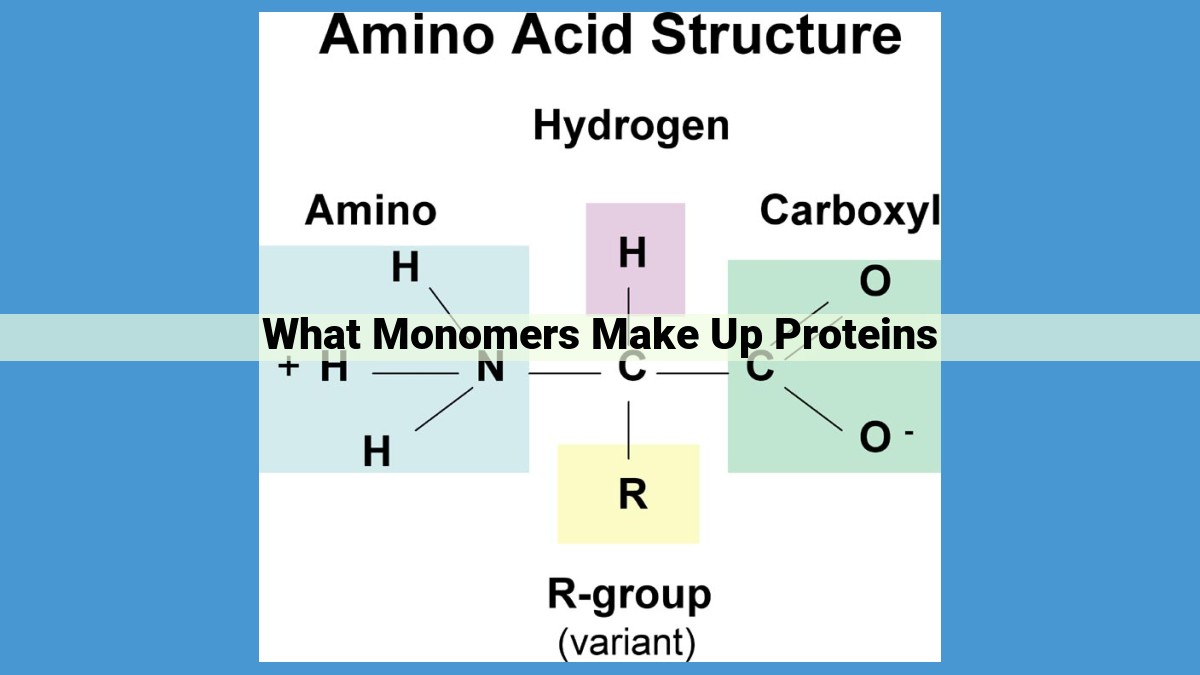
Proteins are composed of monomers called amino acids, which are linked together by peptide bonds. They have a central carbon atom bonded to an amino group, a carboxylic acid group, a side chain, and a hydrogen. The sequence and properties of amino acids determine a protein’s structure and function, with essential amino acids being obtained through diet. Peptide bonds form polypeptides, which can have multiple levels of structure: primary, secondary, tertiary, and quaternary. The correct folding of proteins is crucial for their function and stability, influenced by factors such as amino acid sequence and environmental conditions.
Monomers of Proteins: Amino Acids
Definition of Amino Acids
In the world of biochemistry, amino acids are the fundamental building blocks of proteins. These organic compounds consist of a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (-H), and a unique side chain that varies among different amino acids.
Structure and Properties of Amino Acids
Each amino acid possesses a distinct side chain that determines its chemical properties. These side chains can be nonpolar (water-repelling), polar (water-attracting), or charged (attracted to ions). The arrangement of these side chains along the carbon backbone gives rise to the unique structure and properties of each protein.
Polar and charged amino acids tend to be hydrophilic (water-loving), while nonpolar amino acids are hydrophobic (water-hating). This balance of hydrophilic and hydrophobic regions contributes to the overall solubility and biological functions of proteins.
The Genetic Blueprint of Protein Structure: Unraveling the Connection
Proteins, the workhorses of our cells, play a crucial role in almost every aspect of life. From catalyzing biochemical reactions to transporting molecules, proteins are essential for our survival. But how do these complex molecules acquire their specific shapes and functions? The answer lies in the intricate relationship between genetics and protein structure.
The Genetic Code: The Blueprint for Amino Acids
At the heart of protein structure lies the genetic code, a set of three-nucleotide sequences known as codons. Each codon corresponds to a specific amino acid, the building blocks of proteins. The sequence of codons within a gene determines the order of amino acids in the corresponding protein.
Translation: Bringing the Code to Life
Once the genetic code has been deciphered, the process of translation begins. This molecular machinery converts the linear sequence of codons into a linear sequence of amino acids. Ribosomes, the molecular factories of the cell, read the codons and link the appropriate amino acids together.
As the amino acid chain grows, it begins to fold into a specific three-dimensional conformation. This folding is guided by various factors, including the interactions between different amino acids and the environment inside the cell. The resulting protein structure determines its unique function and properties, making each protein a masterpiece of genetic engineering.
Understanding the genetic basis of protein structure is crucial for biomedical research. By deciphering the genetic code, scientists are paving the way for targeted therapies that can modulate protein function and treat a wide range of diseases. From cancer to neurodegenerative disorders, unraveling the secrets of protein structure holds immense promise for improving human health.
Essential Amino Acids: The Building Blocks of Life
In the intricate tapestry of our bodies, proteins serve as the architects, the catalysts, and the gatekeepers of cellular function. However, not all amino acids, the building blocks of proteins, are created equal. Some, known as essential amino acids, cannot be synthesized by our bodies and must be obtained through our diets.
These irreplaceable nutrients play a pivotal role in our overall health and well-being. They support critical processes such as:
- Tissue growth and repair
- Muscle development
- Hormone production
- Immune system function
- Cognitive function
Dietary Sources of Essential Amino Acids
To ensure optimal intake of these vital nutrients, it is crucial to consume a balanced diet that includes:
Animal Products:
– Meats
– Poultry
– Fish
– Dairy products
– Eggs
Plant-based Sources:
– Quinoa
– Soy products (tofu, tempeh)
– Beans
– Lentils
– Nuts
– Seeds
It is worth noting that while plant-based foods can provide essential amino acids, they often do so in lower amounts or less-optimal combinations. To ensure adequate intake, combining different plant-based sources of protein throughout the day is recommended.
Essential amino acids are the foundation of a healthy body. By consuming a balanced diet that provides sufficient quantities of these nutrients, we empower our bodies to build and repair tissues, maintain muscle mass, produce hormones, and support overall well-being. Remember, essential amino acids are the irreplaceable building blocks that pave the way for a life of vitality and longevity.
Peptide Bonds: The Building Blocks of Polypeptides
In the realm of proteins, the fundamental units are amino acids, which link together through peptide bonds, forming the scaffolding known as polypeptides. Peptides can be thought of as molecular chains, where each amino acid is like a bead strung along the chain.
The formation of peptide bonds is a critical process in protein synthesis. It occurs when two amino acids come together and undergo a condensation reaction, which involves the removal of a water molecule. This reaction creates an amide bond, which is the backbone of every peptide bond.
Enzymes called proteases are responsible for hydrolyzing peptide bonds, breaking the polypeptide chain into smaller fragments or individual amino acids. This hydrolysis reaction is the reverse of condensation and is essential for protein turnover, digestion, and the activation of certain proteins.
Polypeptides can vary greatly in length, ranging from just a few amino acids to hundreds or even thousands. The number of amino acids in a polypeptide determines its molecular weight and complexity. Based on their length, polypeptides can be classified into different types:
- Oligopeptides: Short polypeptides with fewer than 10 amino acids
- Polypeptides: Chains of 10 to 100 amino acids
- Proteins: Long polypeptides with more than 100 amino acids
Proteins perform a vast array of functions within living organisms, including structural support, enzymatic activity, and cellular signaling. The unique structure and composition of each protein are essential for its specific function.
Protein Structure: The Blueprint of Life’s Functions
Like intricate blueprints guiding the construction of majestic buildings, protein structure dictates the diverse functions of these biological workhorses. Understanding the hierarchy of protein structure is akin to unraveling the secrets of how they orchestrate the symphony of life.
Primary Structure: The Foundation
The primary structure forms the backbone of a protein, a linear sequence of amino acids linked by peptide bonds. This chain of amino acids, determined by the genetic code, defines the protein’s identity and serves as the basis for its higher levels of organization.
Secondary Structure: Folding into Patterns
As the polypeptide chain coils and folds, it adopts regular patterns called alpha-helices and beta-sheets. These secondary structures provide stability and shape to the protein, paving the way for more complex interactions.
Tertiary Structure: The Unique Three-Dimensional Fold
The tertiary structure represents the full three-dimensional shape of a protein. It folds into a compact globular form or an extended fibrous shape, creating a precise architecture for specific functions. This intricate folding is driven by interactions between amino acids, such as hydrogen bonding, hydrophobic interactions, and disulfide bonds.
Quaternary Structure: A Collaboration of Units
Some proteins consist of multiple polypeptide chains that assemble into a complex known as the quaternary structure. This arrangement allows for cooperative interactions between subunits, enhancing the protein’s overall function.
Significance of Protein Structure
The structure of a protein is critical for its function. It determines the binding sites for ligands, interacts with other proteins, and enables specific enzymatic reactions. Proper folding is essential for the protein to perform its biological role. Misfolding can lead to loss of function or even disease.
The hierarchy of protein structure, from the primary sequence to the quaternary assembly, unveils the intricate design of these molecular machines. Understanding protein structure not only provides insights into their function but also opens up avenues for manipulating and engineering proteins to address various biological challenges.
Protein Folding and Stability: The Key to Protein Functionality
Proteins, the workhorses of our cells, rely on their precise structure to execute their vital functions. This intricate architecture is achieved through a remarkable process known as protein folding, where a linear chain of amino acids contorts into a specific three-dimensional shape.
Factors Influencing Protein Folding
The folding of proteins is a complex dance influenced by several factors. The amino acid sequence, the building blocks of proteins, plays a crucial role. Amino acids with different chemical properties interact differently, guiding the chain to fold into a specific conformation.
Beyond the amino acid sequence, intramolecular interactions also contribute to the folding process. These interactions include:
- Hydrogen bonding: Forms between polar molecules and plays a vital role in stabilizing protein structures.
- Disulfide bonds: Covalent bonds between cysteine amino acids that provide additional stability and rigidity.
- Ionic bonds: Electrostatic interactions between oppositely charged amino acids.
Influence of the Environment
The environment surrounding a protein can also affect its folding. Factors such as temperature, pH, and the presence of other molecules can influence the folding process. For example, high temperatures can disrupt hydrogen bonding, leading to protein unfolding.
Importance of Proper Folding
Proper folding is essential for protein function. Folded proteins expose functional surfaces that enable them to interact with other molecules, bind ligands, and catalyze chemical reactions. When proteins fail to fold correctly, they often lose their ability to perform their intended tasks.
Consequences of Misfolding
Misfolded proteins can lead to a variety of cellular problems, including:
- Loss of function: Misfolded proteins cannot carry out their normal functions, disrupting cellular processes.
- Protein aggregation: Misfolded proteins can clump together, forming aggregates that can impair cellular function.
- Diseases: Misfolded proteins have been implicated in several diseases, such as Alzheimer’s and Parkinson’s.