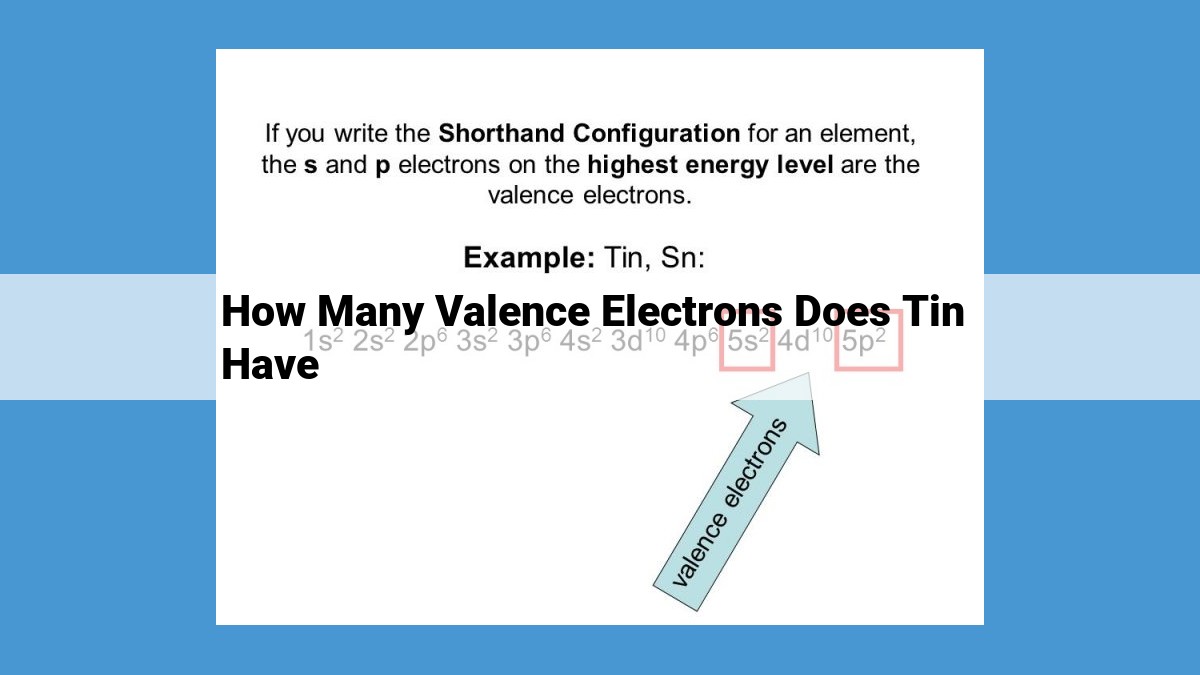
Tin’s Valence Electrons
Tin, located in group 14 of the periodic table, has four valence electrons. As a group 14 element, tin’s valence electron count aligns with the group number. This means tin has four electrons in its outermost electron shell. According to the octet rule, elements strive to gain or lose electrons to achieve a stable configuration with eight valence electrons. Tin’s four valence electrons allow it to participate in various chemical bonds, including ionic and covalent bonds.
Unveiling the Secrets of Valence Electrons: A Journey into the World of Chemistry
Imagine atoms as tiny universes, where valence electrons dance around the nucleus like planets orbiting a star. These electrons hold immense significance in the world of chemistry, as they determine how atoms interact with each other, forming the building blocks of everything around us.
In this blog post, we’ll embark on a fascinating journey to unravel the mystery of valence electrons. We’ll explore their definition, uncover their crucial role in chemical bonding, and understand how they influence the unique properties of elements like tin. Join us as we delve into the captivating world of chemistry.
Defining Valence Electrons: The Gateway to Chemical Bonding
Valence electrons are the outermost electrons in an atom’s electron configuration. They are like the social butterflies of the atomic world, responsible for bonding with neighboring atoms and forming molecules. Their number determines an element’s chemical reactivity, making them the gatekeepers of the chemical reactions that shape our world.
In the language of chemistry, the octet rule plays a central role in understanding valence electrons. This rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons, forming a complete electron shell. This pursuit of stability drives the chemical reactions that create the vast array of substances in our universe.
Tin: A Journey into the Realm of Group 14
Embark on a captivating exploration into the world of tin, a fascinating element that resides within the enigmatic realm of Group 14. As we delve into the properties and characteristics that define tin, we’ll uncover the profound influence of its valence electrons, which play a pivotal role in shaping its chemical destiny.
Tin, a silvery-white metal, boasts a remarkable malleability and ductility, making it highly pliable and workable. Its low melting point and electrical conductivity further enhance its versatility, allowing it to be easily molded and shaped for various industrial applications. Notably, tin possesses a remarkable resistance to corrosion, making it a valuable material for food packaging and household items alike.
As we venture deeper into tin’s atomic structure, we discover its placement as a Group 14 element in the periodic table. This strategic position holds profound implications for tin’s chemical behavior. Valence electrons, the electrons occupying the outermost shell of an atom, determine an element’s chemical properties. Tin, as a Group 14 element, possesses four valence electrons, bestowing it with a unique ability to form chemical bonds and interact with other elements.
The octet rule, a guiding principle in chemistry, states that atoms strive to acquire a stable configuration of eight valence electrons. Tin, with its four valence electrons, readily accepts or shares electrons to achieve this stable arrangement, forming covalent bonds with other atoms. This bonding behavior underlies tin’s diverse chemical interactions and the formation of various tin compounds with a wide range of properties.
Tin’s position within Group 14 not only influences its valence electron count but also its chemical reactivity. Group 14 elements, collectively known as the carbon family, share a gradual increase in metallic character as we descend the group. Tin, positioned towards the heavier end of the group, exhibits a more pronounced metallic character compared to its lighter counterparts. This metallic character imparts tin with enhanced electrical conductivity and malleability, further expanding its potential applications.
**The Periodic Table: A Guide to the Elemental Universe**
Imagine a tapestry woven with the threads of elements, each thread a unique shade representing its distinct properties. The Periodic Table is our guide to this intricate tapestry, a map that organizes these elements based on their atomic number and electron configuration.
The table is arranged in vertical columns, known as groups, each representing a family of elements with similar valence electron counts. These valence electrons are the outermost electrons in an element’s atoms and play a crucial role in determining its chemical behavior.
Each group in the table, from Group 1 (Alkali Metals) to Group 18 (Noble Gases), contains elements with the same number of valence electrons. For example, tin, our protagonist in this tale, belongs to Group 14. This means it possesses four valence electrons, which shape its chemical destiny and metamorphosis into a metalloid.
By understanding the significance of groups in the Periodic Table, we can decipher the fundamental characteristics of elements. We can anticipate the valence electron counts of elements and infer their potential for chemical bonding. Just as a jigsaw puzzle unveils a captivating image when its pieces are assembled, the Periodic Table empowers us to unravel the secrets of the elemental world.
Atomic Number: Unraveling the Identity of Elements
In the realm of chemistry, the atomic number stands as a fundamental property that defines the essence of an element. It is like a unique fingerprint, distinguishing one element from another and paving the way for understanding their distinctive characteristics.
The atomic number represents the number of protons in an atom’s nucleus. These tiny, positively charged particles are the foundation of an element’s identity, as they determine the number of electrons orbiting the nucleus. The number of electrons, in turn, governs an element’s chemical behavior.
The atomic number dictates an element’s position on the periodic table. This iconic chart organizes elements in ascending atomic number, from left to right and top to bottom. Elements with the same atomic number are isotopes of the same element, sharing identical chemical properties but varying in neutron count.
For instance, carbon, with an atomic number of 6, has six protons and six electrons. Its position in the fourth row and first column of the periodic table reflects its four energy levels and one valence electron. This valence electron, located in the outermost shell, determines carbon’s ability to form chemical bonds with other atoms.
In contrast, hydrogen, with an atomic number of 1, has one proton and one electron. It occupies the first position in the periodic table due to its single energy level and valence electron. This unique configuration makes hydrogen highly reactive.
By comprehending the atomic number, we gain insights into the fundamental building blocks of matter. It serves as a guide, helping us navigate the periodic table and unravel the chemical properties of elements. Through this understanding, we can appreciate the intricate tapestry of the chemical world and the role that each element plays in shaping our universe.
Group Number: Unraveling the Code of Valence Electrons
In the realm of chemistry, the periodic table is our guidebook to understanding the behavior of elements. One crucial aspect of this table is the group number, which plays a pivotal role in revealing the number of valence electrons an element possesses.
Valence electrons, the outermost electrons in an atom, are the chemical key to an element’s bonding capabilities. They determine the element’s chemical reactivity and its tendency to form bonds with other elements.
In the periodic table, vertical columns represent groups. Each group houses elements with a similar number of valence electrons. This fundamental characteristic is reflected in their chemical properties and bonding tendencies.
For example, group 1 elements like sodium and potassium have one valence electron, making them highly reactive and prone to forming ionic bonds. Conversely, group 18 elements, known as noble gases, have a stable full complement of valence electrons, rendering them chemically unreactive.
Determining Group Number
Identifying the group number of an element is straightforward. Simply count the number of vertical columns from left to right in which the element resides. This number corresponds to the group number and, consequently, the number of valence electrons.
Tin: A Case in Point
Tin, located in group 14, embodies the principles of group number and valence electrons perfectly. With four valence electrons, tin exhibits a diverse range of chemical properties, forming bonds with both metals and nonmetals. Its versatility as a metalloid stems from this unique electron configuration.
memahami konsep nomor golongan dalam tabel periodik, kita dapat mengekstrapolasi jumlah elektron valensi suatu unsur. Pengetahuan ini penting untuk memprediksi sifat kimia dan reaktivitas unsur, memberikan kita wawasan mendalam tentang dunia yang tersembunyi di dalam atom.