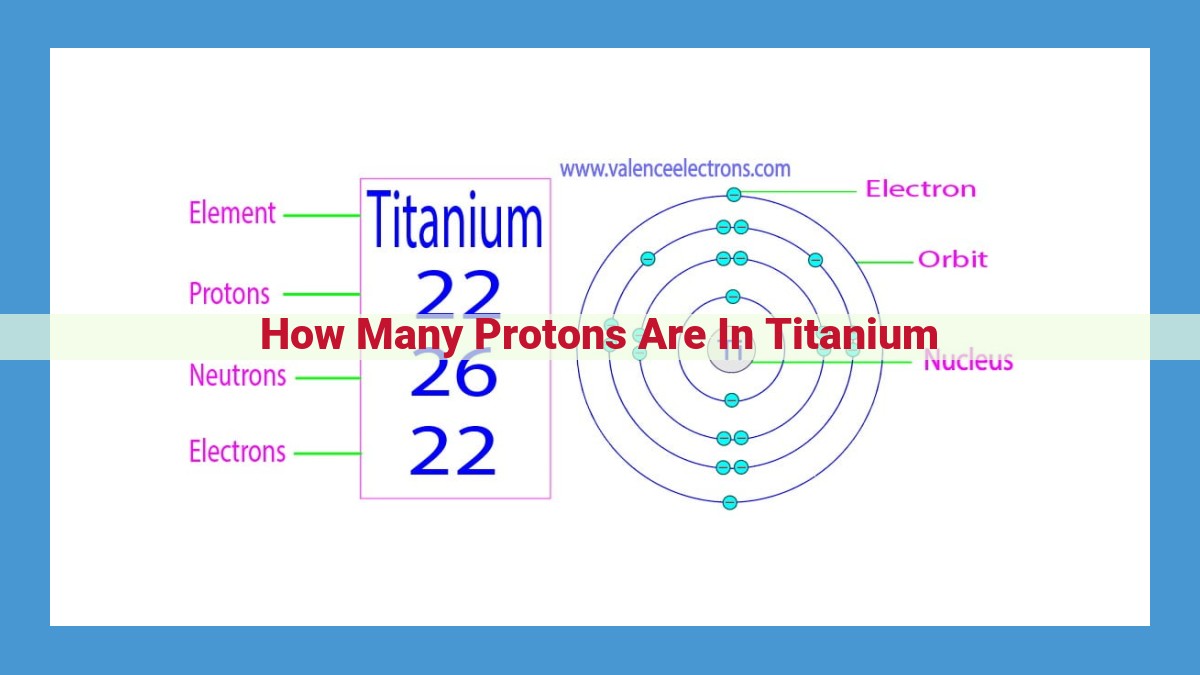
Titanium has an atomic number of 22. The atomic number represents the number of protons in an atom’s nucleus, making titanium’s nucleus contain 22 protons. This number plays a crucial role in determining an element’s identity and chemical properties, shaping titanium’s unique characteristics and applications.
Atomic Number: The Blueprint of Our Elements
In the realm of atoms, the atomic number stands out as a fundamental concept that unlocks the secrets of their nature. It’s the key that defines an element’s identity and determines its specific properties.
Simply put, the atomic number is the number of protons residing within an atom’s nucleus, the heart of the atom. Each element in the periodic table has a unique atomic number, ranging from 1 (hydrogen) to 118 (oganesson). This number dictates the number of electrons that orbit the nucleus, shaping the element’s chemical behavior.
The atomic number governs an element’s chemical characteristics, such as its reactivity, bonding capacity, and the formation of molecules. For instance, all elements with the same atomic number exhibit similar chemical properties, making them part of the same element group in the periodic table.
Understanding the Essence of Atomic Number
The atomic number is tantamount to an element’s DNA, providing the blueprint for its identity and influencing its interactions with other substances. It’s a concept of immense significance in chemistry, allowing scientists to predict the properties of different elements and understand how they form the building blocks of our world.
The Number of Protons: Shaping Chemical Identity and Nuclear Stability
Protons, the fundamental building blocks of atomic nuclei, play a pivotal role in shaping the chemical identity and stability of elements. They not only determine an element’s position on the Periodic Table but also influence their chemical properties and behavior.
Chemical Identity: The number of protons in an atom’s nucleus, known as its atomic number, defines the element to which it belongs. Each element has a unique atomic number that distinguishes it from all others. For instance, hydrogen has one proton, helium two, and oxygen eight. This proton count determines the number of electrons that an atom can accommodate in its electron cloud, which in turn dictates its chemical reactivity and bonding characteristics.
Nuclear Stability: Protons also play a crucial role in maintaining the stability of atomic nuclei. The strong nuclear force, which binds protons and neutrons together, must overcome the repulsive electrostatic force between positively charged protons. For small atoms, the strong force is sufficient to keep the nucleus intact even with a relatively small number of protons. However, as the number of protons increases, the repulsive force becomes more significant, and the nucleus requires more neutrons to maintain stability.
Nucleon Number: A Sum of Subatomic Particles
Unraveling the secrets of atoms involves understanding the fundamental particles that reside at their very core. Nucleon number emerges as a crucial concept in this exploration, representing the total number of protons and neutrons that constitute an atom’s nucleus.
Protons and neutrons form the building blocks of atomic nuclei. Protons carry a positive charge and play a pivotal role in determining an element’s identity. Neutrons, on the other hand, are chargeless and contribute to the stability of the nucleus.
The nucleon number of an atom, often denoted by the symbol A, represents the sum of protons and neutrons present in its nucleus. This number is a fundamental property that distinguishes one atom from another.
Calculating Nucleon Number:
A = Number of protons + Number of neutrons
Understanding the relationship between nucleon number, atomic number (Z), and neutron number (N) is essential. Atomic number refers to the number of protons in the nucleus and determines an element’s position on the periodic table. Neutron number represents the difference between nucleon number and atomic number:
N = A – Z
This relationship highlights the significance of nucleon number in defining the composition and properties of atomic nuclei.
Case Study: Titanium – Unveiling the Secrets of Its Atomic Essence
In the realm of materials science, titanium stands out as an extraordinary element with remarkable properties. To unravel its essence, let’s delve into the depths of its atomic nucleus, where the number of protons dictates its identity and influences its exceptional characteristics.
Titanium, symbolized by the atomic number 22, possesses 22 protons within its nucleus. Protons, the positively charged particles, play a pivotal role in shaping an element’s chemical identity. In titanium’s case, the presence of 22 protons determines its position as the 22nd element on the Periodic Table. It also defines the number of electrons that orbit the nucleus, shaping its chemical reactivity and bonding behavior.
Beyond its chemical nature, the number of protons in titanium’s nucleus also influences its stability. Protons carry a positive charge, creating a strong repulsive force within the nucleus. In order to maintain stability, titanium’s nucleus also contains 26 neutrons, particles with no charge. This delicate balance between protons and neutrons ensures the nucleus remains stable and prevents radioactive decay.
The combination of titanium’s atomic number and neutron number gives rise to a unique set of characteristics that make it highly sought after in various industries. Its strength-to-weight ratio is exceptional, making it ideal for applications where lightweight and durability are paramount, such as aerospace components and medical implants. Additionally, titanium is highly corrosion-resistant, making it suitable for marine environments and chemical processing equipment.
Furthermore, titanium’s atomic number and nucleus structure contribute to its unique biocompatibility. The human body recognizes titanium as inert, allowing it to be used in medical devices and implants without causing rejection or adverse reactions. This remarkable property has revolutionized orthopedic surgery and medical device technology.
Titanium’s journey from its atomic essence to its diverse applications showcases the intricate interplay between subatomic particles and the macroscopic properties they confer upon materials. By understanding the fundamental building blocks of this exceptional element, we gain a deeper appreciation for its unique characteristics and the technological advancements it enables.