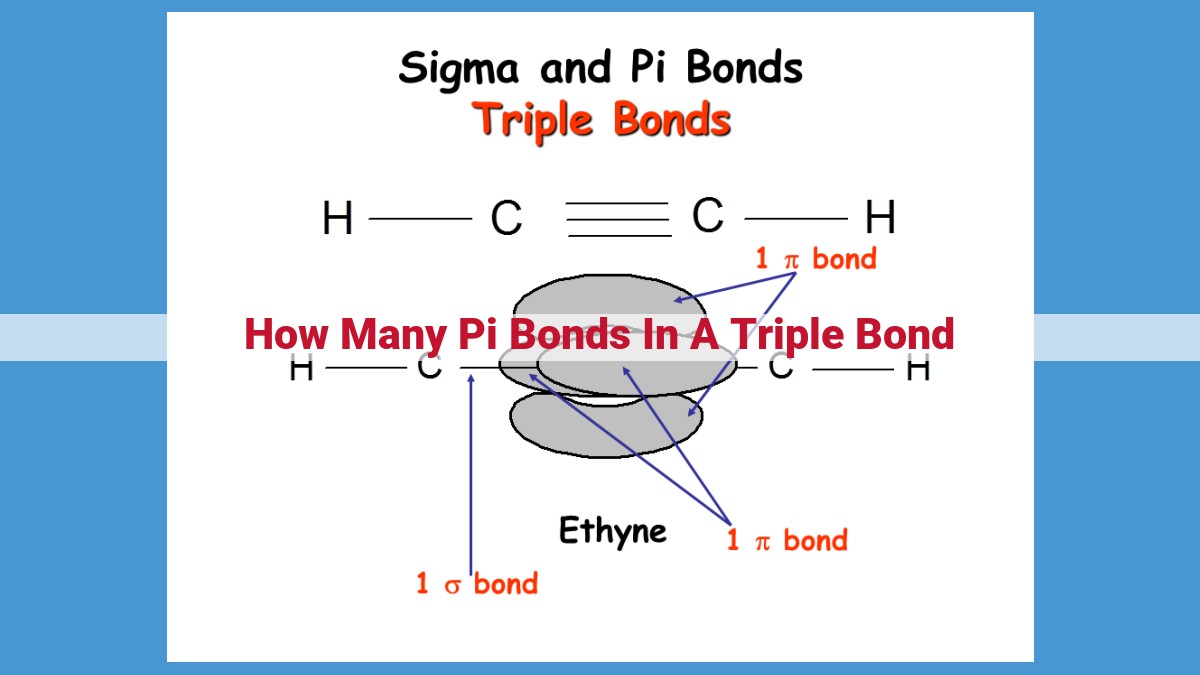
In a triple bond, which consists of three pairs of shared electrons between atoms, there are two pi bonds in addition to one sigma bond. Pi bonds are formed by the lateral overlap of p-orbitals and are weaker than sigma bonds. In a triple bond, the sigma bond is formed by the head-on overlap of hybridized atomic orbitals, while the pi bonds are formed by the overlap of unhybridized p-orbitals.
- Define a triple bond as a type of covalent bond with three pairs of electrons shared between two atoms.
- Explain the concept of multiple bonds and their relation to triple bonds.
Triple Bonds: The Strongest Covalent Connections
In the realm of chemistry, bonds between atoms are the fundamental forces that hold molecules together. Among these bonds, triple bonds stand out as the strongest and most unique. Join us as we delve deeper into the world of triple bonds and explore their remarkable properties.
Defining Triple Bonds and Multiple Bonds
Triple bonds are a type of covalent bond that involves three pairs of electrons shared between two atoms. These bonds are represented by the triple symbol “≡”. Covalent bonds occur when atoms share electrons to achieve stability, and triple bonds represent the strongest form of this sharing.
The concept of multiple bonds stems from the idea that some atoms can form bonds involving more than one pair of electrons. Single bonds involve the sharing of one pair of electrons, while double bonds involve two pairs. Triple bonds, with their three pairs of shared electrons, represent the ultimate expression of covalent bonding.
Delving into the Realm of Carbon-Carbon Triple Bonds: Alkynes and Their Fascinating Properties
In the realm of chemistry, bonds between atoms play a crucial role in shaping the structure and properties of molecules. One captivating type of bond is the triple bond, characterized by its robust nature as it comprises three pairs of electrons shared between two atoms. Triple bonds are a testament to the versatility of carbon atoms, forming the backbone of a fascinating group of compounds known as alkynes.
Alkynes: The Triple Bond Champions
Alkynes are organic compounds that contain at least one carbon-carbon triple bond. These molecules are distinguished by their unique properties, and the simplest alkyne, ethyne (C2H2), is widely known as acetylene. Other common alkynes include propene (C3H4) and butyne (C4H6), each showcasing the increasing number of carbon atoms in the chain. These compounds serve as the elemental building blocks for a diverse range of chemical substances.
Hybridization: The Key to Triple Bond Formation
Understanding the nature of triple bonds requires venturing into the realm of hybridization, a concept that describes how atomic orbitals combine to form molecular orbitals. In the case of triple bonds, the carbon atoms involved undergo sp hybridization, a process that results in the formation of three equivalent hybrid orbitals. These orbitals are oriented in a linear fashion, pointing directly towards each other. The sp hybridization allows for the formation of one sigma bond and two pi bonds, giving rise to the distinctive triple bond.
Unveiling the Sigma and Pi Bonds
The triple bond consists of one sigma bond, which is formed by the head-to-head overlap of the sp hybrid orbitals, and two pi bonds, which are formed by the lateral overlap of p-orbitals. The sigma bond provides the primary strength to the bond, while the pi bonds contribute additional stability. The presence of pi bonds in triple bonds bestows unique characteristics upon these molecules, setting them apart from their single and double bond counterparts.
Pi Bonds: The Sideways Overlappers
Pi bonds, formed by the sideways overlap of p-orbitals, exhibit a weaker nature compared to sigma bonds. However, their presence profoundly influences the properties of molecules. They are responsible for the restricted rotation around triple bonds, a phenomenon that arises due to the perpendicular orientation of the p-orbitals involved in the pi bonds. This restricted rotation has a significant impact on the chemical reactivity and stereochemistry of alkynes.
Formation of Pi Bonds: A Tale of Overlapping Orbitals
The formation of pi bonds in triple bonds is an intricate process that involves the overlap of p-orbitals oriented perpendicular to the sigma bond. This process can be visualized as the p-orbitals rotating and approaching each other until they overlap, leading to the formation of two pi bonds. The strength of the pi bonds depends on the extent of the overlap between the p-orbitals, and the presence of substituents can influence the pi bond strength and orientation.
The triple bond, a remarkable covalent bond formed by the sharing of three pairs of electrons between two atoms, is a cornerstone of alkyne chemistry. Through the concept of sp hybridization, carbon atoms adopt a linear geometry, facilitating the formation of one sigma bond and two pi bonds. These pi bonds, resulting from the sideways overlap of p-orbitals, impart distinct properties to alkynes, including restricted rotation and unique reactivity patterns. Understanding the nature of triple bonds is essential for comprehending the behavior and applications of these versatile organic compounds.
Hybridization of Carbon Atoms in Triple Bonds
In the realm of chemistry, bonds between atoms determine the molecular structure and properties of the resulting compounds. Among the various types of covalent bonds, the triple bond stands out as a captivating subject. This blog post delves into the intricate details of carbon-carbon triple bonds, their structure, and the fascinating concept of hybridization.
sp Hybridization and the Triple Bond
Imagine carbon atoms dancing in a captivating waltz, their orbitals intertwining and aligning in a specific manner. In the case of a triple bond, the carbon atoms undergo sp hybridization, creating three hybrid orbitals with a unique blend of s and p orbital characteristics. These sp hybrid orbitals form the backbone of the triple bond, facilitating the sharing of six electrons between the two carbon atoms.
Linear Shape: A Consequence of sp Hybridization
As the sp hybrid orbitals form, they arrange themselves in a linear fashion, aligning directly opposite each other. This arrangement leads to the characteristic linear shape of triple bonds, as seen in molecules like ethyne and propene. The linear geometry results from the optimized overlap of the hybrid orbitals, minimizing repulsion between the electron pairs and ensuring maximum bond strength.
Bridging the Gap: Sigma and Pi Bonds
Within the triple bond, two distinct types of bonds emerge: sigma and pi bonds. The sigma bond, formed by the head-on overlap of the sp hybrid orbitals, constitutes the backbone of the bond, providing the primary structural support. However, the excitement doesn’t end there. Superimposed on the sigma bond are two pi bonds_, formed by the lateral overlap of p orbitals perpendicular to the sigma bond axis. These pi bonds, weaker than the sigma bond, contribute significantly to the overall bond strength and shape the electronic properties of the molecule.
Sigma and Pi Bonds in Triple Bonds
In the realm of chemistry, bonds are the glue that holds atoms together, creating molecules and shaping the world around us. Among these bonds, the triple bond stands out as a special type of covalent bond, where three pairs of electrons are shared between two atoms. To delve deeper into the nature of triple bonds, let’s explore the concept of sigma and pi bonds.
A sigma bond is a strong, head-on overlap of orbitals, where the electron density is concentrated directly between the nuclei of the bonded atoms. In a triple bond, there is only one sigma bond.
Pi bonds, on the other hand, are weaker and result from the sideways overlap of orbitals. They are formed when p-orbitals, which have a dumbbell shape, overlap parallel to the sigma bond. In a triple bond, there are two pi bonds.
The presence of three bonds, one sigma and two pi, in a triple bond gives it its unique properties. The sigma bond provides the backbone of the bond, while the pi bonds add extra strength and stability. This combination results in a bond that is both strong and short, allowing for a linear molecular geometry.
**Pi Bonds: The Weaker Link in Triple Bonds**
In the realm of chemical bonds, there exists a fascinating dance between atoms as they share their precious electrons. Among the most intriguing of these bonds is the triple bond, where three pairs of electrons dance effortlessly between two atoms. But within this triple bond, there’s a tale of two bonds: the robust sigma bond and the enigmatic pi bond.
Let’s delve into the world of pi bonds and unravel their unique characteristics. Pi bonds, unlike their sigma counterparts, arise from a sideways overlap of p-orbitals. These p-orbitals, shaped like dumbbells, dance around the atoms’ nuclei. When they align perpendicular to the sigma bond, they initiate the formation of a pi bond.
This sideways overlap gives pi bonds their distinct properties. They’re weaker than sigma bonds. Why? Because their overlap is less extensive. The p-orbitals, with their “dumbbell shape,” don’t completely overlap, leaving a small gap between them. This gap translates into a weaker bond.
But don’t underestimate the pi bond’s significance. Its very existence amplifies the strength of the triple bond. Together with the two sigma bonds, the pi bond creates a formidable partnership that gives triple bonds their exceptional stability.
Pi bonds also play a vital role in determining the shape of molecules. Their sideways overlap forces the atoms into a linear configuration, with the atoms aligned in a straight line. This unique shape allows triple bonds to exist in molecules like alkynes, such as ethyne and propene.
So, there you have it—the intriguing tale of pi bonds, the weaker but essential partners in the triple bond dance. They may not be as strong as sigma bonds, but their sideways overlap adds a unique twist to the molecular world, influencing molecular shape and stability in ways that continue to fascinate chemists.
Formation of the Pi Bonds in Triple Bonds
Covalent bonds between atoms can form in various ways, with triple bonds being one of the strongest types. A triple bond involves the sharing of three pairs of electrons between two atoms. While sigma bonds are formed from head-on overlap of orbitals, pi bonds are created from sideways overlap of p-orbitals.
In the case of a triple bond, one sigma bond and two pi bonds combine to form the overall bond. The sigma bond results from the overlap of the sp hybrid orbitals of the carbon atoms involved. These orbitals are formed by the hybridization of one s-orbital and two p-orbitals of each carbon atom, resulting in a linear arrangement of the atoms.
The pi bonds are created by the overlap of unhybridized p-orbitals perpendicular to the sigma bond orbital. These p-orbitals contain one electron each and overlap sideways, creating two regions of electron density above and below the plane formed by the sigma bond.
The overlap of the p-orbitals in the pi bonds is less efficient than the head-on overlap in sigma bonds. This results in weaker pi bonds compared to sigma bonds. However, the presence of two pi bonds in a triple bond provides additional stability, contributing to the overall strength of the bond.
Visual Representations of Pi Bond Formation
[Image: Formation of Pi Bonds in a Triple Bond]
Additional Notes for SEO Optimization:
- Use relevant keywords such as “triple bond,” “pi bond,” “sigma bond,” “hybridization,” and “carbon atom.”
- Include alt text for images that describes the content of the image.
- Consider using subheadings to break up the text into smaller sections for easier reading.
- Optimize the blog post for mobile devices by using responsive design.