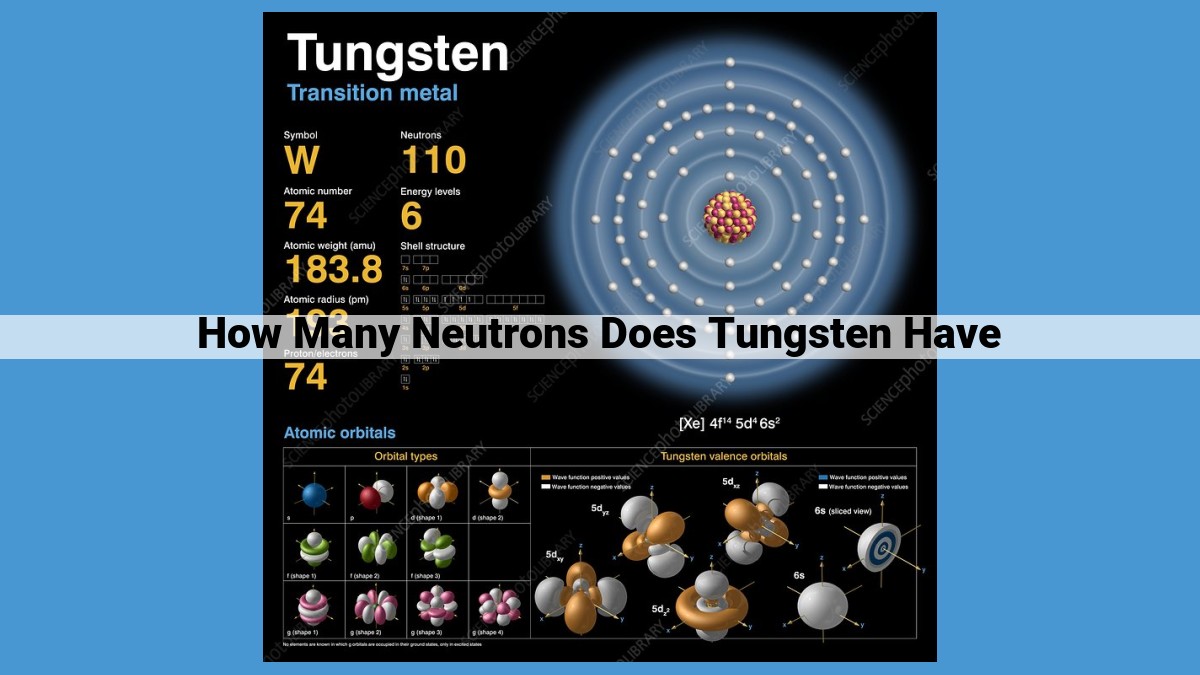
Tungsten’s neutron number, a measure of the number of neutrons in its atomic nucleus, plays a crucial role in its atomic structure and properties. Tungsten’s atomic number (74) represents the number of protons, while the neutron number varies among its isotopes. The most common isotope, 184W, has 110 neutrons. Neutron number influences an isotope’s mass, stability, and properties. Mass spectrometry techniques determine the neutron number by measuring mass-to-charge ratios. Understanding the neutron number is essential for applications in nuclear energy, medical imaging, and more.
- Explain the concept of neutron number and its significance in atomic structure.
- State the purpose of the blog post: to determine the neutron number of tungsten.
Understanding the Neutron Number of Tungsten: A Journey into Atomic Structure
In the realm of atomic structure, every element possesses unique properties that define its identity and behaviour. Among these properties, the neutron number holds profound significance in shaping the very essence of an atom. This blog post embarks on a journey to unravel the neutron number of tungsten, a remarkable element renowned for its extraordinary properties.
The neutron number represents the number of neutrons within the nucleus of an atom. Neutrons, along with protons and electrons, are fundamental particles that determine the composition and characteristics of matter. The neutron number plays a pivotal role in determining the stability, mass, and even radioactive properties of an atom.
Our quest to uncover the neutron number of tungsten necessitates a deeper understanding of atomic structure. Tungsten, denoted by the symbol W, is an element with an atomic number of 74. This atomic number signifies the presence of 74 protons in its nucleus, balanced by 74 electrons orbiting around it. However, the number of neutrons in the nucleus can vary, giving rise to different isotopes of tungsten.
Discovering the Secrets of Tungsten: Understanding its Atomic Structure
In the realm of chemistry, every element holds a fascinating story to tell. Today, we embark on a journey to unravel the mysteries of tungsten, a remarkable element known for its exceptional properties.
Tungsten: A Titan of the Periodic Table
Tungsten, denoted by the symbol W, stands as the 74th element in the periodic table. Its atomic number, the number of protons in its nucleus, is 74, making it a heavy metal. Tungsten atoms possess 74 electrons, which orbit the nucleus in distinct energy levels.
Delving into the Atomic Architecture of Tungsten
At the heart of every tungsten atom lies a compact nucleus, composed of protons and neutrons. The number of protons, equal to the atomic number, determines an element’s identity. Neutrons, on the other hand, are neutral particles that contribute to the atom’s mass.
In tungsten atoms, the dance between protons and electrons creates a delicate balance. The positive charge of the protons is neutralized by the negative charge of the electrons, resulting in an electrically neutral atom.
The Significance of Neutron Number
The neutron number of an atom, the number of neutrons in its nucleus, plays a critical role in its stability and properties. Isotopes of an element are atoms with the same atomic number but different neutron numbers. These variations in neutron number lead to variations in mass and can influence an isotope’s behavior.
Unveiling Tungsten’s Isotopic Tapestry
Tungsten boasts a rich isotopic landscape, with 184W emerging as the most common isotope. 184W contains 74 protons and 110 neutrons, giving it a neutron number of 110. Other tungsten isotopes, such as 182W and 186W, have neutron numbers of 108 and 112, respectively.
The Impact of Neutron Number in Tungsten
The neutron number profoundly affects tungsten’s atomic nucleus, influencing its stability and properties. Tungsten isotopes with higher neutron numbers tend to be more stable, resisting radioactive decay. This stability makes them valuable for applications in nuclear energy and medical imaging.
Understanding the neutron number of tungsten is essential for unraveling the complexities of this remarkable element. Its variation among isotopes gives rise to a spectrum of properties, unlocking its potential for diverse applications. From the depths of its atomic structure to its practical uses, tungsten continues to fascinate scientists and engineers alike, inspiring advancements in various fields.
Chapter 3: Neutron Number and Isotopes – Unveiling Tungsten’s Nuclear Secrets
Isotopes: The Versatile Siblings
In the atomic world, elements like tungsten don’t exist in isolation. They have family members called isotopes. Isotopes are atoms of the same element with identical atomic numbers but different neutron numbers. This subtle variation in neutron number creates a fascinating dance of similarities and differences among isotopes.
Neutron Number’s Influence on Atomic Symphony
Neutrons, the unsung heroes of the atomic nucleus, play a crucial role in shaping the destiny of isotopes. By varying the neutron count, isotopes possess distinct masses. This mass variation directly impacts the physical and chemical properties of each isotope. It’s like a cosmic orchestra, where each neutron subtly alters the melody of the element’s behavior.
**Determining the Neutron Number of Tungsten: Unraveling the Secrets of Its Atomic Structure**
Measuring Neutron Number through Mass Spectrometry: A Scientific Adventure
In the realm of chemistry, understanding the intricacies of atomic structure is crucial. One key aspect is the neutron number, which unveils important information about an atom’s composition and behavior. To uncover the neutron number of tungsten, we embark on a scientific journey using a remarkable technique: mass spectrometry.
Mass spectrometry is a sophisticated tool that allows us to measure the mass-to-charge ratios of atoms. In our quest to determine tungsten’s neutron number, we harness this technique’s ability to accurately measure an atom’s mass number. The mass number, the sum of protons and neutrons in an atomic nucleus, provides us with a crucial stepping stone in our mission.
As we delve into the fascinating world of tungsten, we discover its symbol, W, and its atomic number of 74. This atomic number tells us the number of protons in tungsten’s nucleus. Equipped with this knowledge, we can now use mass spectrometry to determine the mass number of tungsten’s isotopes.
By analyzing the mass-to-charge ratios of tungsten’s isotopes, we can deduce their respective mass numbers. Subtracting the atomic number (74) from the mass number reveals the neutron number, the long-sought-after piece of information.
Neutron Number in Tungsten Isotopes
- List the known isotopes of tungsten and their respective neutron numbers.
- Highlight the most common isotope, 184W, and its neutron number of 110.
Neutron Number in Tungsten Isotopes
Tungsten, a transition metal with the symbol W and atomic number 74, exhibits diverse properties due to variations in its neutron number. The neutron number, which represents the number of neutrons in an atom’s nucleus, significantly influences the mass and characteristics of tungsten isotopes.
Tungsten has 18 known isotopes, each with a distinct neutron number. The atomic mass of an isotope is calculated by adding the numbers of protons (atomic number) and neutrons. For instance, the most abundant isotope, 184W, has 74 protons (atomic number) and 110 neutrons, resulting in an atomic mass of 184.
Other known isotopes of tungsten include:
- 180W with 106 neutrons
- 182W with 108 neutrons
- 183W with 109 neutrons
These isotopes vary in their stability and properties based on their neutron number. For example, 184W has a relatively long half-life and is stable for practical purposes. In contrast, isotopes with significantly different neutron numbers, such as 180W and 186W, have shorter half-lives and are radioactive.
The variation in neutron number among tungsten isotopes has practical implications. The stable isotope, 184W, is widely used in various applications. Its high melting point and high density make it suitable for manufacturing filaments, electrodes, and welding rods. In contrast, radioactive isotopes of tungsten find applications in medical imaging and nuclear energy.
Understanding the neutron number is crucial for predicting the stability and properties of tungsten’s atomic nucleus. This knowledge enables scientists and engineers to tailor tungsten isotopes for specific applications, contributing to advancements in various fields.
Significance of Neutron Number in Tungsten
The neutron number, representing the count of neutrons within an atom’s nucleus, plays a pivotal role in shaping the stability and defining the properties of tungsten’s atomic nucleus. Tungsten, symbolized by W, is a chemical element with an atomic number of 74, indicating it possesses 74 protons within its nucleus. The protons contribute a positive charge, while the neutrons remain neutral, serving as a balancing force against the protons’ positive charge.
The neutron number impacts the mass and stability of tungsten isotopes. Isotopes are variations of the same element that share the same number of protons but differ in neutron number. These variations influence the atomic mass and overall properties of the isotopes. A higher neutron number generally corresponds to a heavier isotope.
In the case of tungsten, the most prevalent isotope is 184W with a neutron number of 110. This isotope comprises approximately 31% of natural tungsten and forms the foundation for various applications. Other isotopes of tungsten exist, each with a distinct neutron number, contributing to a range of properties and uses.
The significance of neutron number extends beyond its influence on atomic stability. Tungsten isotopes find widespread applications in diverse fields:
- Nuclear Energy: Tungsten isotopes with specific neutron numbers are utilized in nuclear reactors as neutron moderators, controlling the rate of nuclear reactions.
- Medical Imaging: Tungsten isotopes, particularly 188W, are employed in medical imaging techniques, such as single-photon emission computed tomography (SPECT), to diagnose and monitor various medical conditions.
In summary, the neutron number serves as a crucial factor in understanding tungsten’s atomic structure, influencing its stability and properties. The variations in neutron number among tungsten isotopes result in distinct applications, spanning from nuclear energy to medical imaging, highlighting the profound importance of neutron number in the world of chemistry and beyond.