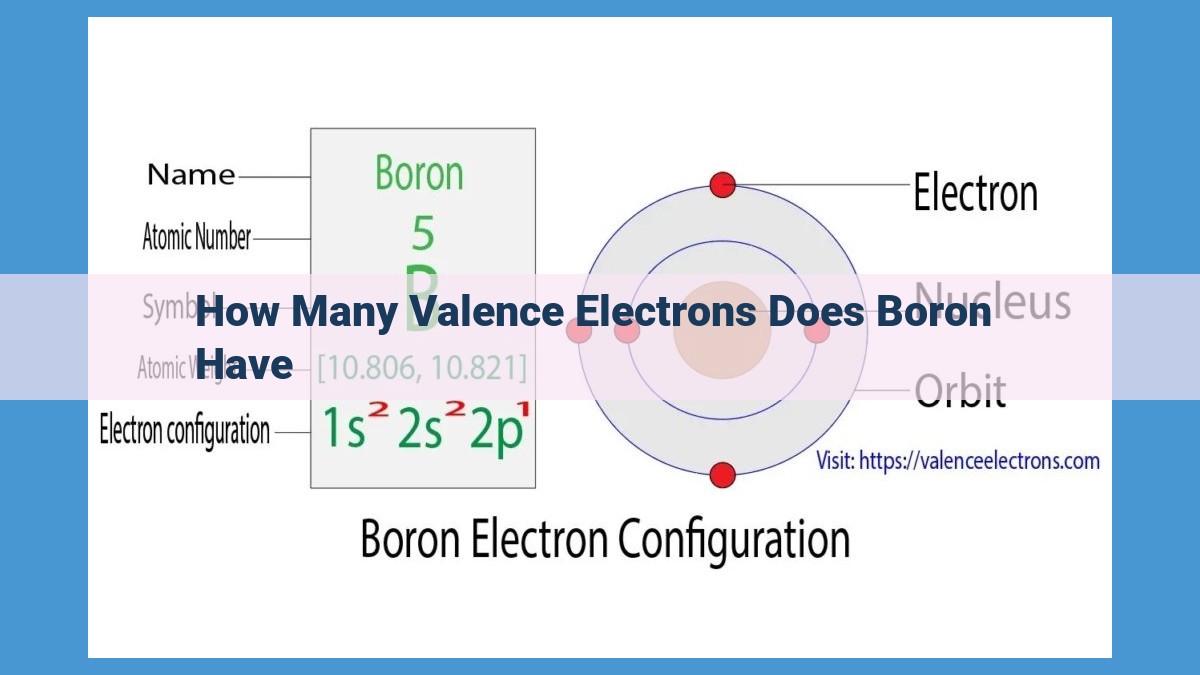
Boron, a metalloid element in Group 13 with an atomic number of 5, has one valence electron. The valence shell, consisting of orbitals n=2, contains the atom’s valence electrons, which dictate its chemical behavior. Boron’s electronic configuration, 1s2 2s2 2p1, reveals that it possesses a single electron in its valence shell, the 2p orbital. This solitary valence electron plays a crucial role in determining boron’s reactivity and ability to form chemical bonds.
Valence Electrons: The Key Players in Chemical Reactions
In the vast realm of chemistry, the concept of valence electrons holds immense significance. These electrons, residing in the outermost shell of an atom, are the pivotal players that determine how atoms interact with each other, paving the way for chemical reactions.
Defining Valence Electrons and Their Importance
Valence electrons are the electrons that occupy the highest energy level of an atom. They are the most loosely held electrons and, therefore, the most reactive. When atoms come into close proximity, their valence electrons can be exchanged or shared, resulting in the formation of chemical bonds.
By understanding the number and behavior of valence electrons, chemists can predict the chemical properties of elements and the types of bonds they can form. These electrons are the driving force behind chemical reactivity, shaping the molecular landscape of our universe.
Boron: A Metalloid’s Electronic Enigma
In the realm of chemistry, electrons play a pivotal role, shaping the interactions and reactions between elements. Valence electrons, those residing in the outermost shell of an atom, hold the key to understanding an element’s chemical behavior. Enter boron, a metalloid element whose unique electronic configuration sets it apart from its peers.
Boron occupies Group 13 of the periodic table, a position that reveals its atomic number of 5. The atomic number, a defining characteristic of each element, determines the total number of electrons within its structure. Thus, boron possesses 5 electrons.
Its position in Group 13 further hints at its electronic arrangement. Group 13 elements typically have 3 valence electrons, but boron defies this norm with only 1 valence electron. This anomaly stems from its electronic configuration of 1s² 2s² 2p¹. The superscript numbers denote the number of electrons occupying each orbital within each energy level. Boron’s valence electron resides in the 2p¹ orbital, the outermost energy level.
The Valence Shell of Boron: A Chemical Journey
In the realm of chemistry, the valence shell holds a vital role in shaping the chemical properties of elements. It’s like a stage, where electrons play out their dance of bonding and reactions. Let’s delve into the valence shell of boron, an enigmatic metalloid that holds a unique place on the periodic table.
The valence shell, also known as the outermost shell, is the highest energy level of an atom’s electron configuration. It’s the electrons in this shell that determine how an element reacts with others, forming the bonds that create molecules and compounds.
Within the valence shell, electrons reside in specific orbitals. Orbitals are like tiny regions of space where electrons are most likely to be found. In boron’s case, its valence shell consists of a single 2p orbital. This means that boron has only one electron in its valence shell, a lone ranger ready to interact with the world.
The number of valence electrons is a crucial factor in determining an element’s chemical behavior. Boron’s single valence electron makes it highly reactive, as it eagerly seeks to complete its valence shell by gaining or sharing electrons. This unique characteristic drives boron’s ability to form bonds with a wide range of elements, creating compounds with diverse properties.
Dissecting Boron’s Electronic Configuration: A Journey Into Chemical Understanding
In the realm of chemistry, the dance of electrons holds the key to unlocking the secrets of chemical reactions. Valence electrons, the outermost electrons of an atom, play a pivotal role in shaping an element’s chemical behavior. Meet boron, a fascinating metalloid element, whose valence electrons hold intriguing tales to tell.
Boron resides in Group 13 of the periodic table, a strategic position that grants it an atomic number of 5. This number dictates the total number of electrons within boron’s atomic structure.
Every atom possesses a valence shell, an energy level that houses the valence electrons. These electrons occupy specific regions called orbitals. In boron’s case, its valence shell consists of a single orbital, designated as 2p.
To decipher boron’s electronic configuration, we employ a notation that meticulously describes the distribution of electrons across its orbitals. For boron, this configuration reads: 1s² 2s² 2p¹.
Breaking down this cryptic notation, we uncover the secrets of boron’s electron distribution. The 1s² portion indicates two electrons occupying the innermost energy level (1s). The 2s² portion reveals two more electrons in the next energy level (2s). Finally, the 2p¹ portion unveils the presence of a lone electron residing in the valence shell’s 2p orbital.
This single valence electron is the key to understanding boron’s chemical properties. Its unpaired nature makes it highly reactive, eager to form bonds with other atoms. This characteristic fuels boron’s ability to participate in a diverse array of chemical reactions, shaping its behavior and applications in the world of chemistry.
Determining Boron’s Valence Electrons: A Journey into the Atomic Realm
In the fascinating world of chemistry, the concept of valence electrons reigns supreme. These electrons, occupying the outermost shell of an atom, play a pivotal role in determining the chemical characteristics and bonding behavior of an element. Let’s embark on a journey to unravel the secrets of boron’s valence electrons.
Boron, an intriguing metalloid element, finds its place in Group 13 of the periodic table. Its atomic number, 5, reveals that it possesses a total of five electrons. These electrons are distributed among the various energy levels or shells surrounding the atomic nucleus.
The valence shell, the outermost electron shell, is the key to understanding an element’s chemical properties. It is here that valence electrons reside, occupying orbitals within the shell. In boron’s case, the valence shell is the second energy level (n=2).
To determine the number of valence electrons in boron, we must count the electrons present in the valence shell orbitals. Boron’s valence shell configuration is 2p1, indicating the presence of a single electron in the 2p orbital.
Therefore, we can confidently conclude that boron has one valence electron. This solitary electron determines the chemical behavior of boron, influencing its ability to form bonds and react with other elements.
Understanding the number of valence electrons is crucial for predicting an element’s chemical reactivity. It provides valuable insights into the formation of chemical bonds, the stability of compounds, and the overall behavior of an element in chemical reactions.