Carbon’s six electrons occupy its 1s (2), 2s (2), and 2p (2) orbitals. Its atomic number, 6, indicates that carbon has six protons and six electrons. According to the Pauli Exclusion Principle, no two electrons can have the same quantum state. Hund’s Rule dictates that electrons occupy orbitals with parallel spins before pairing. Analyzing carbon’s electron configuration reveals two unpaired electrons in the 2p orbitals, contributing to carbon’s unique chemical versatility.
Unveiling Carbon’s Electronic Secrets: A Journey into Its Electron Configuration
In the vast expanse of chemistry, carbon stands as a cornerstone element, its unique properties shaping the very fabric of life. But what lies at the heart of carbon’s remarkable nature? It’s its electron configuration, a dance of electrons within orbitals that unlocks its chemical versatility.
Peeling Back the Layers of Carbon’s Electron Orchestra
Carbon, with its atomic number 6, boasts six electrons. These electrons waltz around the nucleus, occupying different energy levels known as orbitals. Carbon’s electron configuration can be visualized as:
- 1s²: Two electrons reside in the closest orbital to the nucleus, designated as 1s.
- 2s²: Another pair of electrons occupies the slightly farther 2s orbital.
- 2p²: The final two electrons find their home in the 2p orbitals, which exist in three perpendicular orientations: 2pₓ, 2pᵧ, and 2p₂, each capable of holding one electron.
The Importance of Atomic Number: A Tale of Equal Partners
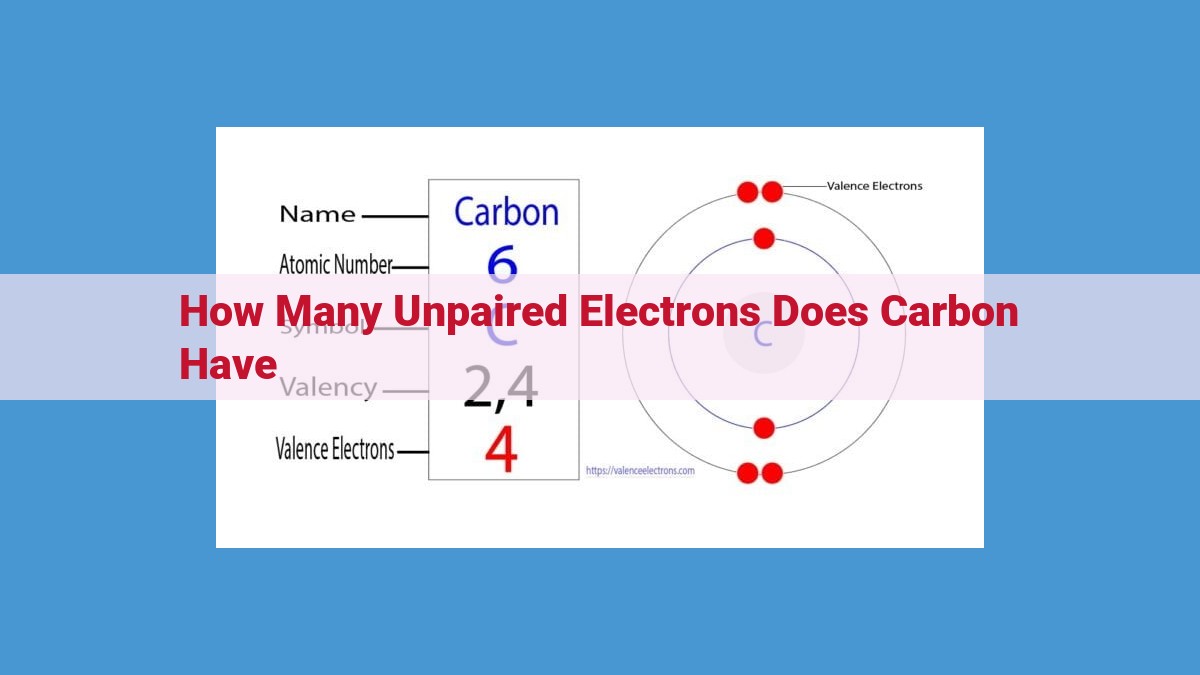
The atomic number of an element, including carbon, reveals the number of protons within its nucleus. Since atoms are electrically neutral, the number of electrons that orbit the nucleus must also equal the number of protons. Hence, carbon’s atomic number of 6 indicates the presence of six protons and six electrons.
Enter the Pauli Exclusion Principle: A Quantum Dance of Individuality
The Pauli Exclusion Principle adds a twist to the electron configuration story. It declares that no two electrons can share the exact same quantum state, a combination of energy level, orbital shape, and spin direction. In essence, each electron must have its own unique identity within the atom.
Hund’s Rule: A Quantum Waltz of Magnetism
Hund’s Rule further governs the electron configuration of carbon. It states that when electrons occupy orbitals of the same energy level (in this case, the 2p orbitals), they prefer to do so with parallel spins rather than pairing up. As a result, carbon’s two 2p electrons align their spins in the same direction, creating two unpaired electrons.
Unveiling Carbon’s Unpaired Electrons: The Key to Its Chemical Prowess
These unpaired electrons in carbon’s 2p orbitals are crucial for its chemical reactivity. They allow carbon to form covalent bonds with other atoms, sharing electrons to create a vast array of molecules that make up our world. From organic compounds found in living organisms to inorganic materials like carbon dioxide, the unpaired electrons of carbon play a pivotal role.
Carbon’s electron configuration, with its six electrons distributed among orbitals and guided by quantum rules, lies at the foundation of its unique properties. The unpaired electrons in its 2p orbitals empower carbon with its versatility, enabling it to dance with other atoms and orchestrate the formation of countless molecules. It’s a testament to the fascinating world of quantum chemistry, where the arrangement of electrons dictates the behavior and properties of the elements that shape our universe.
The Significance of Carbon’s Atomic Number
Every atom in the universe possesses a unique fingerprint, known as its atomic number. It’s the passport that reveals the identity and behavior of each element. For carbon, the building block of life, its atomic number holds a pivotal role in shaping its chemical characteristics.
Carbon’s atomic number is 6, which is a clue to its electron configuration. Atoms possess equal numbers of protons (positively charged particles in the nucleus) and electrons (negatively charged particles orbiting the nucleus). Thus, carbon’s 6 protons are matched by 6 electrons.
These electrons reside in specific energy levels or orbitals around the nucleus, arranged in layers called shells. The first shell, closest to the nucleus, can hold up to 2 electrons, while the second shell can accommodate 8.
Carbon’s 6 electrons are distributed as follows:
- 2 electrons in the first shell (1s orbital)
- 4 electrons in the second shell (2s and 2p orbitals)
The 2s orbital is filled with 2 electrons, while the 2p orbital holds the remaining 2 electrons. This distribution is crucial because it determines carbon’s chemical reactivity.
The presence of 2 unpaired electrons in the 2p orbital makes carbon a versatile partner in forming bonds with other atoms. These unpaired electrons can form covalent bonds, sharing electrons with other atoms, creating the myriad molecules that make up the world around us.
Carbon’s atomic number, therefore, plays a fundamental role in shaping its chemical behavior. It governs the number of electrons and their arrangement in orbitals, ultimately determining carbon’s ability to form bonds and its status as the cornerstone of organic chemistry and life itself.
Unveiling the Secrets of the Pauli Exclusion Principle: A Quantum Odyssey
In the realm of quantum mechanics, the dance of electrons within atoms becomes a fascinating tale of forbidden relationships and entangled destinies. The Pauli Exclusion Principle, a fundamental law governing the behavior of electrons, orchestrates this dance by dictating that no two electrons can share the exact same quantum state.
Imagine a bustling party, where each guest embodies an electron. The Pauli Exclusion Principle resembles a strict party host, enforcing a rule that no two guests can occupy the same table. Each table represents a unique quantum state, characterized by distinct properties such as energy level and spin direction.
This principle ensures the diversity of the atomic world. Without it, electrons would crowd into the lowest energy state, creating a monotonous and uniform existence. The Pauli Exclusion Principle forces electrons to seek unique identities, distributing them across the available tables (quantum states), creating the rich tapestry of atomic properties we observe.
In the case of carbon, with its six electrons, the Pauli Exclusion Principle dictates the precise filling of its energy levels. The two 1s orbitals, the lowest in energy, accommodate two electrons each, their spins antiparallel to comply with another quantum principle. The remaining four electrons occupy the 2s and 2p orbitals. Here, the Pauli Exclusion Principle prohibits two electrons from sharing the same spin direction within a single orbital.
Thus, the two 2p orbitals each house two electrons, but with parallel spins. This arrangement has profound implications for carbon’s chemistry, granting it the unique ability to form diverse bonds and shape the world around us.
The Pauli Exclusion Principle is an enigmatic force that shapes the very fabric of our universe. It orchestrates the intricate dance of electrons, giving rise to the remarkable diversity of atoms and the countless wonders of chemistry.
Applying Hund’s Rule to Carbon’s Electronic Structure
In the realm of quantum chemistry, Hund’s rule reigns supreme as the guiding force for electron distribution within atoms. This rule dictates that when filling orbitals of equal energy, electrons will do so with parallel spins before pairing up.
Imagine carbon, our humble building block of life, standing at the crossroads of orbital occupancy. With its six electrons eager to find their rightful place, Hund’s rule steps in to orchestrate the dance.
Carbon’s electronic configuration, symbolized as 1s²2s²2p², reveals a tale of orbital occupancy. The first two electrons settle comfortably into the 1s orbital, followed by two more in the 2s orbital. However, the remaining four electrons face a tantalizing choice, the 2p orbitals.
According to Hund’s rule, these unpaired electrons will initially occupy separate 2p orbitals with parallel spins. Parallel spins occur when electrons spin in the same direction, depicted as arrows pointing either up or down. This arrangement maximizes the electron’s magnetic moment, creating a more stable and energetically favorable configuration.
In carbon’s case, two electrons occupy each of the three 2p orbitals, with one electron spinning up and the other spinning down. This arrangement yields two unpaired electrons in carbon, setting the stage for its remarkable chemical versatility.
These unpaired electrons become the key players in carbon’s chemistry, allowing it to form diverse bonds with other atoms. The story of Hund’s rule and its impact on carbon’s electron configuration is a testament to the intricate ballet that governs the electron world.
Determining the Number of Unpaired Electrons in Carbon
Carbon’s unique electronic structure plays a pivotal role in its extraordinary ability to form bonds with a vast array of elements, creating the building blocks of life and countless other compounds. At the heart of this versatility lies the number of unpaired electrons in carbon’s outermost orbitals.
Examining carbon’s electron configuration (1s² 2s² 2p²) reveals that its two unpaired electrons reside in the degenerate 2p orbitals. These electrons have parallel spins, a phenomenon governed by Hund’s rule.
The presence of these unpaired electrons is of paramount importance in carbon’s chemistry. They allow carbon to form four covalent bonds, sharing electrons with other atoms to achieve a stable octet configuration. This bonding capability enables carbon to create a multitude of compounds, ranging from simple molecules like methane to complex biomolecules like DNA.
In summary, carbon’s two unpaired electrons in its 2p orbitals are crucial for its unparalleled bonding versatility, making it the cornerstone of organic chemistry and a vital element for life as we know it.