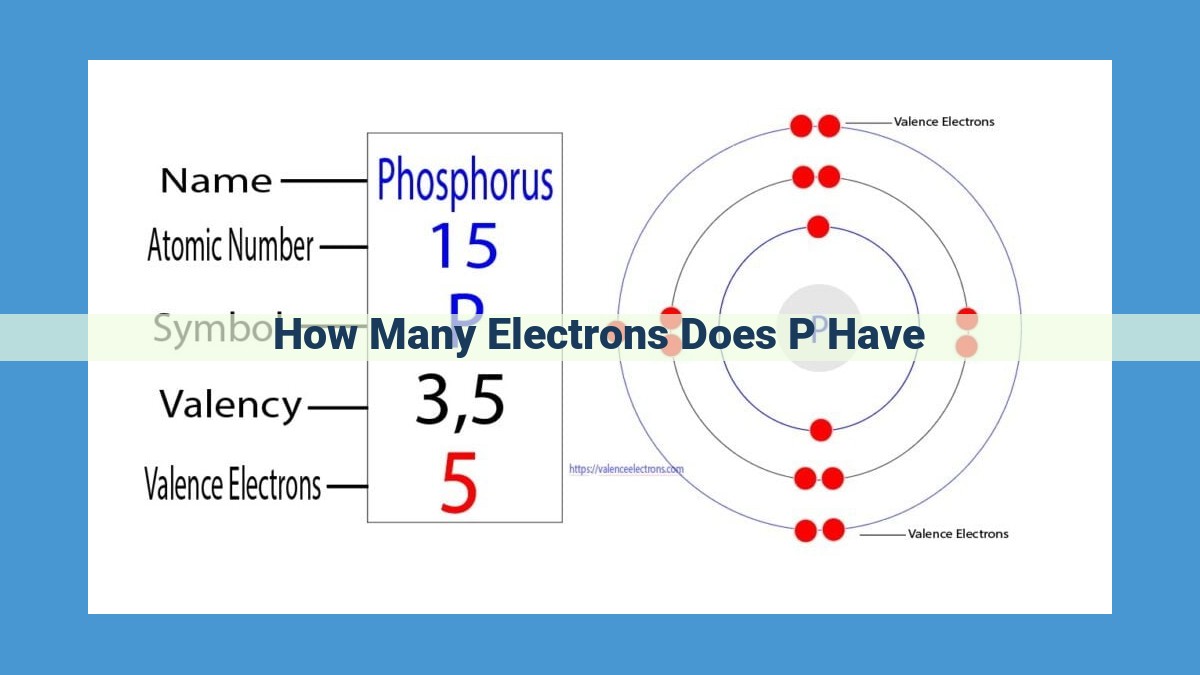
Phosphorus possesses 15 protons and 15 electrons. Its electron configuration is 1s²2s²2p⁶3s²3p³. This configuration depicts the arrangement of electrons in energy levels: two in the first, two in the second, six in the third, and two in the fourth energy level. The number of valence electrons, those in the outermost energy level, plays a crucial role in phosphorus’s chemical properties.
Electrons: Essential Components of Matter
- Discuss the charge, subatomic nature, and orbital behavior of electrons.
Electrons: The Essential Building Blocks of Matter
Electrons, the negatively charged subatomic particles, are the fundamental components of all matter. These tiny particles reside in orbitals, whirling around the atom’s nucleus at incredible speeds. Electrons play a crucial role in defining an atom’s chemical properties and determine how it interacts with other atoms.
Each electron carries a specific and fundamental charge that is inseparable from its existence. This charge, a key characteristic of electrons, allows them to interact with other charged particles, such as protons and neutrons.
Electrons are not simply passive inhabitants of the atom; they engage in a constant dance of motion. Their orbitals, akin to miniature solar systems, govern their movement within the atom. These orbitals come in various shapes and sizes, creating a complex electronic structure that influences an atom’s behavior.
The presence of electrons is essential for the formation of atoms and molecules. They participate in chemical reactions, determining the properties of substances and the ways in which they interact with each other. Understanding the nature and behavior of electrons is paramount in unraveling the mysteries of matter and exploring the fundamental workings of our universe.
Protons: The Positive Force in the Atomic World
In the intriguing realm of subatomic particles, protons play a crucial role in shaping the very fabric of matter. These tiny yet mighty particles, residing in the heart of every atom, serve as the positive counterweights to the negatively charged electrons. Join us on a journey to unravel the mysteries of protons, their fascinating properties, and their profound influence on the elements that make up our world.
The Electric Guardians
Protons are positively charged particles, carrying an equal and opposite charge to the electron’s negative charge. This inherent polarity is the driving force behind the electromagnetic force, the fundamental interaction that governs the behavior of charged particles. Protons attract electrons, forming the strong bonds that hold atoms together. Without the presence of protons, electrons would scatter aimlessly, rendering matter unstable and formless.
Subatomic Nature and Nuclear Abode
Protons are subatomic particles, meaning they exist within the atom’s nucleus alongside neutrons. While electrons occupy the space around the nucleus, protons and neutrons form the atom’s dense, central core. Protons are not elementary particles but rather composed of even smaller building blocks called quarks. Three quarks, specifically two up quarks and one down quark, combine to create a single proton.
Atomic Number: The Identity Fingerprint
The number of protons in an atom’s nucleus defines its atomic number. This unique identifier distinguishes one element from another. Each element in the periodic table has a specific atomic number, which determines its chemical properties and behavior. By understanding the atomic number, scientists can identify and classify the vast array of elements that constitute our universe.
Neutrons: Mass Contributors with No Charge
In the bustling universe of atoms, there exists a subatomic particle that while carrying no electric charge, plays a crucial role in shaping their very existence: neutrons. These enigmatic constituents, devoid of electrical personality, reside within the atomic nucleus alongside their positively charged partners, protons.
Delving into the Neutron’s Nature
Neutrons, as their name suggests, are electrically neutral particles with a mass slightly greater than that of protons. Unlike their charged counterparts, neutrons are composed of three fundamental particles known as quarks. These quarks, in a perpetual dance of quantum fluctuations, form a tightly bound trio that grants neutrons their mass and electrical neutrality.
Location and Role within the Nucleus
Nestled snugly within the atom’s nucleus, neutrons form a dense core alongside the positively charged protons. This central region, housing the bulk of an atom’s mass, is the heart of its identity. The interplay between protons and neutrons determines the atom’s unique characteristics and its position on the periodic table.
Mass Number and Isotopes
The mass number of an atom, expressed as A, represents the total number of protons and neutrons within its nucleus. While the number of protons defines the element’s identity, the number of neutrons can vary, resulting in isotopes. Isotopes of the same element share the same atomic number but differ in their mass numbers, a consequence of their varying neutron counts. This variation in neutron content influences an element’s physical and chemical properties, giving rise to the rich diversity of elements we observe in the natural world.
Atomic Number: A Fingerprint for Elements
Every substance that surrounds us, from the air we breathe to the food we eat, is composed of tiny building blocks known as atoms. These atoms are the fundamental units of matter, and they are made up of even smaller subatomic particles: electrons, protons, and neutrons.
Protons: The Positive Center
At the heart of every atom lies its nucleus, where protons, positively charged particles, reside. The number of protons inside the nucleus is what distinguishes one element from another. This unique number is known as the atomic number.
Electrons: The Negative Counterbalance
Balancing out the positive protons are electrons, negatively charged particles that orbit the nucleus. Electrons are arranged into specific energy levels, with each level holding a certain number of electrons. The number of electrons in an atom is always equal to the number of protons, resulting in a нейтральный overall charge.
Neutron: Neutral Contributors
Together with protons, neutrons occupy the nucleus. Unlike protons and electrons, neutrons have no electrical charge, hence their name. They primarily contribute to an atom’s mass. The number of neutrons can vary within an element, giving rise to different isotopes.
The Fingerprint of Identity
The atomic number plays a crucial role in determining the chemical identity of an element. It reveals the element’s unique number of protons, which in turn dictates the number of electrons. These electrons are responsible for the element’s chemical properties and behavior.
For instance, let’s consider phosphorus, an element with an atomic number of 15. This means that a phosphorus atom contains 15 protons and 15 electrons. By understanding its atomic number, we can deduce its distinctive chemical characteristics and predict how it will interact with other elements.
The atomic number serves as an invaluable tool in chemistry. It is the key to identifying elements, comprehending their electronic structure, and predicting their chemical behavior. By understanding this concept, we gain a deeper appreciation for the fundamental building blocks of our world.
Phosphorus: A Case Study in Electron Configuration
To fully understand the fascinating world of atomic structure, let’s embark on a journey to explore the electrons, protons, and neutrons that make up every atom. These fundamental particles play crucial roles in defining the identity and properties of all matter.
Electrons, Protons, and Neutrons
Electrons: These tiny, negatively charged particles reside outside the atomic nucleus, occupying specific energy levels or orbitals. They determine an atom’s chemical behavior.
Protons: Located in the nucleus, protons have a positive charge that balances the electrons’ negative charge. The number of protons defines an element’s identity, assigning it a unique atomic number.
Neutrons: Also found in the nucleus, neutrons are uncharged particles that contribute to an atom’s mass but do not affect its chemical properties.
Atomic Number: A Unique Fingerprint
Each element has a distinctive atomic number, which is simply the number of protons in its nucleus. A phosphorus atom has an atomic number of 15, meaning it contains 15 protons.
Electron Configuration: Phosphorus in the Spotlight
Now, let’s dive into the electron configuration of phosphorus. This describes the arrangement of its electrons in energy levels.
- First Energy Level: Holds 2 electrons.
- Second Energy Level: Accommodates 8 electrons.
- Third Energy Level: Hosts the remaining 5 electrons, making phosphorus a valency electron element.
Phosphorus has a total of 15 electrons, with 5 in its outermost energy level. These valence electrons determine how phosphorus interacts with other atoms in chemical reactions.
Knowing the electron configuration of phosphorus allows us to understand its chemical properties and behavior. It’s an essential tool for chemists and a fascinating insight into the building blocks of our universe.