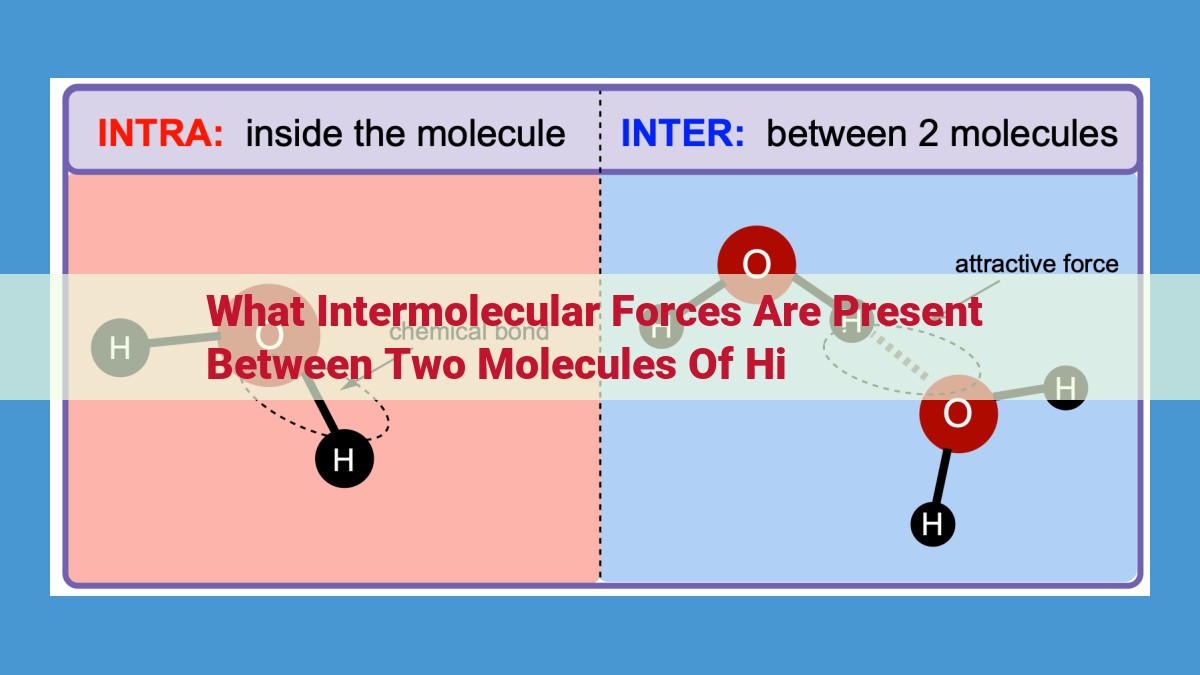
Intermolecular forces (IMFs) are attractive forces that act between molecules. The type of IMF present between two molecules of HI is hydrogen bonding. Hydrogen bonding is a strong IMF that occurs between molecules containing a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In the case of HI, the hydrogen atom is bonded to iodine, which is a relatively electronegative atom. This results in a partial positive charge on the hydrogen atom and a partial negative charge on the iodine atom, creating a dipole. The dipole of one HI molecule can interact with the dipole of another HI molecule, forming a hydrogen bond.
- Definition and significance of IMFs
- Types of IMFs
Intermolecular Forces: The Glue that Holds Matter Together
In the microscopic world of molecules, tiny forces play a crucial role in determining the behavior and properties of matter. These intermolecular forces (IMFs) are the invisible bonds that connect molecules and give substances their distinct characteristics.
IMFs are classified into three main types: hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Each type arises from different interactions between molecules and varies in strength. Hydrogen bonding, the strongest IMF, occurs when hydrogen atoms are bonded to highly electronegative atoms, such as oxygen, nitrogen, or fluorine. This creates a strong dipole moment, resulting in powerful intermolecular attraction.
Dipole-dipole interactions are weaker than hydrogen bonding but stronger than London dispersion forces. They arise between molecules that have permanent dipole moments due to uneven distribution of electrons. These dipole moments align and attract each other, forming intermolecular bonds.
London dispersion forces, the weakest IMF, exist between all molecules, regardless of their polarity. They arise from the continuous movement of electrons, creating temporary fluctuations in electron density. These fluctuations induce opposite dipoles in neighboring molecules, leading to weak intermolecular attraction.
The strength of IMFs has a profound impact on the physical properties of substances. Substances with strong IMFs, like water, have high melting points, boiling points, and viscosity. This is because the strong intermolecular forces make it harder to overcome the molecular interactions that hold the substance together. Substances with weaker IMFs, like gases, have low melting points, boiling points, and viscosity, as their intermolecular forces are too weak to hold the molecules in a fixed arrangement.
IMFs are essential for understanding the behavior of molecules and predicting the properties of substances. They play a crucial role in a wide range of phenomena, from the behavior of liquids and solids to the binding of biological molecules. By understanding IMFs, scientists can gain insights into the structure, function, and properties of matter at the molecular level.
Hydrogen Bonding: The Invisible Glue That Governs Our World
In the intricate tapestry of nature, the dance of molecules weaves a symphony of interactions that define the properties of the world around us. Among these molecular masquerades, hydrogen bonding stands as a key choreographer, shaping the behavior of substances, influencing their properties, and fostering remarkable phenomena.
Hydrogen Bonding: An Atomic Embrace
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. The electronegative atom hogs the electron cloud, creating a partial positive charge on the hydrogen atom, while the electronegative atom becomes partially negative.
These oppositely charged poles attract each other, forming a strong dipole-dipole force. Unlike ordinary dipole-dipole interactions, hydrogen bonding is directional, meaning the attraction occurs along a specific axis. This directional nature gives hydrogen bonds their unique ability to form stronger intermolecular bonds than other types of dipole-dipole forces.
The Strength and Formation of Hydrogen Bonds
The strength of a hydrogen bond depends on several factors, including the electronegativity difference between the two atoms involved, the distance between the atoms, and the solvent environment. The greater the electronegativity difference, the shorter the distance, and the more nonpolar the solvent, the stronger the hydrogen bond.
Hydrogen bonds can form between molecules or within the same molecule (intramolecular hydrogen bonding). They can also form chains or networks, creating a web of interactions that influences the physical and chemical properties of substances.
Applications of Hydrogen Bonding in the Real World
Hydrogen bonding plays a crucial role in numerous natural and industrial processes. Here are a few examples:
-
Water: The unique properties of water, such as its high melting point, boiling point, and viscosity, are attributed to the extensive hydrogen bonding between water molecules. Hydrogen bonding also allows water to dissolve many polar substances and is essential for biological processes such as protein folding.
-
DNA and RNA: The double helix structure of DNA and RNA is stabilized by hydrogen bonds between complementary base pairs. These hydrogen bonds provide the genetic code with its remarkable stability.
-
Polymers: Hydrogen bonding is responsible for the strength and resilience of many synthetic polymers, such as nylon and polyurethane. Hydrogen bonds hold the polymer chains together, giving them durability and resistance to heat and chemicals.
Hydrogen bonding is a fundamental intermolecular force that governs the behavior of substances at the molecular level. Its directional nature, strength, and versatility make it an essential force in shaping the physical properties, chemical reactions, and biological processes of our world. Understanding hydrogen bonding is crucial for chemists, biochemists, physicists, and anyone seeking to unravel the secrets of the molecular world.
Dipole-Dipole Interactions: A Dance of Opposing Poles
Introduction:
In the realm of molecular interactions, the captivating dance of dipole-dipole forces plays a vital role in shaping the physical properties and behavior of substances. Let’s delve into this captivating interplay between polar molecules.
Understanding Dipole-Dipole Forces:
Imagine polar molecules like tiny magnets, carrying a positive and negative pole. These molecules possess uneven electron distribution, creating a separation of charges within the molecule. The positive pole of one molecule aligns itself with the negative pole of another, forming a feeble yet significant attraction called a dipole-dipole force.
Formation and Strength of Dipole-Dipole Forces:
The strength of dipole-dipole forces largely depends on the magnitude of the molecular dipole moment. A higher dipole moment indicates a greater separation of charges, resulting in stronger dipole-dipole interactions. Additionally, the distance between interacting molecules affects force strength; as distance increases, the attraction weakens.
Examples and Significance of Dipole-Dipole Interactions:
Water, the elixir of life, is a prime example of dipole-dipole interactions. The polarity of water molecules allows them to form hydrogen bonds, the strongest type of dipole-dipole interaction. These interactions influence water’s unique physical properties, including its high boiling point and remarkable ability to dissolve ionic compounds.
Dipole-dipole forces also play a critical role in protein folding. The alignment of charged amino acid residues within proteins creates dipole moments, stabilizing protein structure and enabling its diverse functionalities within the body.
Conclusion:
Dipole-dipole interactions are a fundamental force that governs the behavior of polar molecules, influencing their physical properties and shaping the molecular world. From the intricate dance of water molecules to the precise folding of proteins, these forces play a pivotal role in the symphony of chemical interactions that shape our universe.
London Dispersion Forces: The Invisible Glue Holding Your World Together
In the realm of molecules, there exists a quiet force that silently governs their behavior and shapes our world in countless ways. London dispersion forces are the subtle yet omnipresent interactions between nonpolar molecules, holding them together like an invisible glue.
These forces arise from the ceaseless dance of electrons within molecules. Even in nonpolar molecules, where electrons are evenly distributed, there’s a slight and temporary fluctuation in their distribution, creating tiny moments of uneven charge. These momentary induced dipoles interact with each other, forming weak yet persistent attractions called London dispersion forces.
The strength of these forces depends on the size and polarizability of the molecule. Larger and more polarizable molecules have more dispersed electrons, leading to stronger induced dipoles and hence, stronger London dispersion forces. This explains why heavier noble gases like xenon have higher boiling points than lighter ones like helium, as the larger electron clouds in xenon facilitate stronger London dispersion forces.
Examples of London dispersion forces abound in everyday life. They’re responsible for the cohesion of wax, the adhesion of gecko feet to walls, and even the formation of clouds in the sky. They play a crucial role in the behavior and properties of liquids and solids, affecting their melting and boiling points, viscosity, and surface tension. Understanding London dispersion forces is essential for comprehensive knowledge of molecular interactions and their impact on the macroscopic world we experience.
Strength and Comparison of Intermolecular Forces
As we explore the realm of intermolecular forces (IMFs), it’s crucial to understand their relative strength and the factors that influence it. IMFs fall into three primary categories: hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Their ranking in terms of strength is:
-
Hydrogen Bonding: The strongest IMF, arises from the strong electrostatic attraction between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom.
-
Dipole-Dipole Interactions: Weaker than hydrogen bonding, these interactions arise between polar molecules that possess a permanent dipole moment, meaning they have a separation of positive and negative charges.
-
London Dispersion Forces: The weakest IMF, present in all substances, result from temporary, instantaneous distortions in electron clouds that create temporary dipoles.
The strength of IMFs is influenced by several factors, including:
- Polarity: More polar molecules exhibit stronger IMFs.
- Molecular Size: Larger molecules tend to have stronger IMFs due to a greater surface area for interaction.
- Shape: Molecules with more symmetrical shapes tend to have weaker IMFs.
Significance of IMF Strength
The strength of IMFs has a profound impact on the physical properties of substances. Stronger IMFs generally result in:
- Higher melting points: More energy is required to overcome the strong IMFs and separate the molecules.
- Higher boiling points: More energy is required to vaporize the liquid, as the IMFs must be broken.
- Higher viscosity: The stronger IMFs hinder the flow of molecules past each other, making the liquid more viscous.
Understanding the strength and comparison of IMFs is critical for predicting the behavior of molecules and materials in various applications, such as drug design, crystal engineering, and surface science.
The Invisible Hand: Intermolecular Forces and Their Impact on Everyday Life
Unveiling the Hidden Symphony of Molecules
In the grand orchestra of matter, molecules dance to the rhythm of intermolecular forces (IMFs). These invisible forces, acting like ethereal threads, govern the behavior of molecules, shaping their physical properties and influencing their myriad interactions.
IMFs: The Architects of Physical Properties
Among the many effects of IMFs, their profound impact on melting point, boiling point, and viscosity stands out. The strength of IMFs determines the energy required to overcome these forces and transition between solid, liquid, and gas phases.
Melting Point: The Dance of Solids
IMFs hold solid molecules tightly in place, forming a rigid network. Stronger IMFs require higher temperatures to overcome, resulting in higher melting points. For instance, water’s strong hydrogen bonds require a high melting point of 0°C, while iodine’s weak London dispersion forces allow it to melt at a mere 114°C.
Boiling Point: A Measure of Volatility
The strength of IMFs also influences the boiling point of a substance. Stronger IMFs require higher temperatures to break the intermolecular bonds and release molecules into the gas phase. This explains why water boils at a relatively high 100°C, while hydrocarbons like propane boil at much lower temperatures due to their weaker London dispersion forces.
Viscosity: The Resistance to Flow
IMFs play a pivotal role in determining the resistance of a liquid to flow. Stronger IMFs result in higher viscosity, making the liquid less likely to flow easily. Honey’s thick consistency is a testament to its strong hydrogen bonding, while the low viscosity of gasoline stems from its weak London dispersion forces.
Real-Life Applications: A Triumph of Understanding
The impact of IMFs extends far beyond academic laboratories and into the realm of everyday life. From the performance of lubricants to the properties of solvents, IMFs guide the behavior of materials in countless applications:
- Lubricants: Strong IMFs ensure that lubricants maintain their viscosity under high pressures and temperatures, preventing metal-on-metal contact.
- Solvents: IMFs dictate the ability of solvents to dissolve other substances. Water’s strong hydrogen bonds make it an excellent solvent for polar molecules like sugar, while nonpolar solvents like benzene dissolve nonpolar substances like oil.
- Drug Design: Understanding IMFs is crucial for designing drugs that interact effectively with biological molecules. Hydrogen bonding and dipole-dipole interactions are essential for drug-receptor binding and the development of targeted therapies.
Intermolecular forces, though invisible to the naked eye, orchestrate the intricate dance of matter around us. Their strength and character shape the physical properties of substances, from the melting of ice to the flow of blood. Understanding IMFs is not merely an academic pursuit but a window into the fascinating tapestry of life itself.