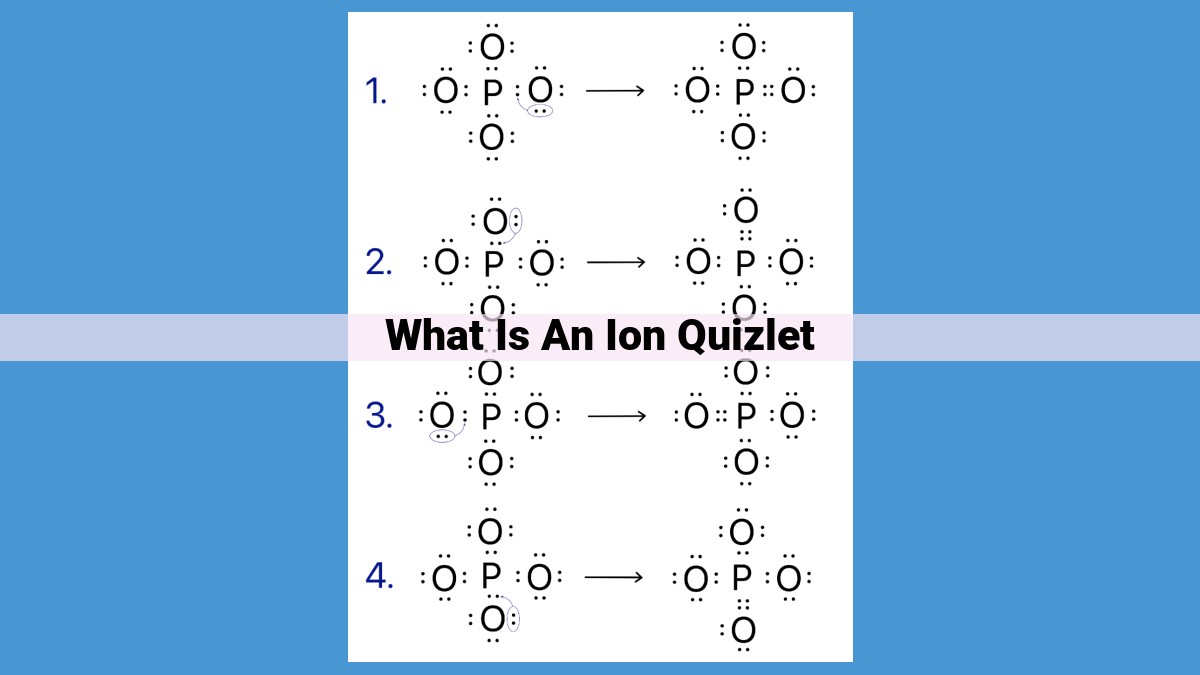
- What is an Ion?
An ion is an atom or molecule that has lost or gained one or more electrons, giving it an electric charge. Ions are classified as cations (positive charge) or anions (negative charge) depending on the type of charge gained or lost. The process of forming ions is called ionization, which can occur through various mechanisms influenced by factors like ionization energy and the number of valence electrons.
Ions: The Building Blocks of Matter
In the realm of chemistry, ions reign supreme as essential building blocks of matter. They dance around us, shaping the world in ways we often fail to notice. So, let’s dive into the fascinating universe of ions and unravel their secrets.
What are Ions?
Imagine a neutral atom with a harmonious balance of electrons and protons. When this equilibrium is disrupted, ions are born. If an atom loses electrons, it becomes a positively charged cation. On the flip side, if it gains electrons, it transforms into a negatively charged anion.
These charged particles don’t just exist in isolation. They exist in nature as compounds, interacting with other ions and neutral atoms to form a myriad of molecules and substances that make up everything around us.
Cations: The Positively Charged Ions
Cations are the positive dudes in the ion family. They’re formed when an atom sheds one or more of its outer electrons, more specifically, its valence electrons.
Valence electrons are like the social butterflies of an atom, frolicking in the outermost energy level. When an atom loses a valence electron, it becomes positively charged because it has more protons than electrons.
Think of a sodium atom. It has one lonely valence electron, which it’s eager to get rid of. When it does, it becomes a sodium ion, denoted as Na+.
Anions: The Negatively Charged Ions
In contrast to cations, anions are the negatively charged ions. They’re formed when an atom gains one or more valence electrons, increasing its overall electron count.
Here’s where the magic happens. When an atom gains valence electrons, it becomes negatively charged because it now has more electrons than protons.
Take a chlorine atom, for example. It has seven valence electrons, and it’s always looking for one more to complete its outer energy level. When it finds a willing donor, it gains an electron and transforms into a chloride ion (Cl-).
Cations: Positively Charged Ions
Meet the Positives: Cations
In the world of ions, there are two main players: cations and anions. Cations are the positively charged members of the ion family, carrying a positive electrical charge. They’re formed when an atom loses one or more valence electrons, the electrons hanging around the outer shell of the atom.
The Loss of Valence Electrons
Valence electrons are like the social butterflies of the atom. They’re the ones interacting with other atoms to form chemical bonds. When an atom loses a valence electron, it gains a positive charge, becoming a cation. This process is called ionization.
Common Cations
Some common cations include:
- Sodium (Na+): Found in table salt and helps regulate nerve impulses.
- Potassium (K+): Vital for heart function and muscle contractions.
- Calcium (Ca2+): Essential for bone formation and muscle function.
- Magnesium (Mg2+): Involved in over 300 enzyme reactions in the body.
- Hydrogen (H+): A key component of acids and plays a role in many chemical reactions.
Cations are crucial players in maintaining the body’s electrical balance and performing essential functions for life. Understanding how these positively charged ions are formed is key to unraveling the intricacies of chemistry and the human body.
Anions: Negatively Charged Ions
What are Anions?
Anions are atoms or molecules carrying an excess of electrons, granting them a negative charge. They form when an atom or molecule gains one or more extra electrons. This electronic surplus shifts the balance of charges, making the anion negatively charged.
Formation of Anions
Anions form when an atom or molecule accepts valence electrons from other atoms or ions. Valence electrons are those located in the outermost energy level of an atom, which are responsible for chemical bonding.
The gain of these electrons changes the atomic or molecular structure, resulting in the formation of an anion. The number of valence electrons gained determines the magnitude of the negative charge.
Common Anions
Numerous anions exist in nature, each with its unique structure and properties. Some common anions include:
- Chloride (Cl-)
- Bromide (Br-)
- Iodide (I-)
- Fluoride (F-)
- Sulfate (SO42-)
- Carbonate (CO32-)
- Nitrate (NO3-)
- Phosphate (PO43-)
Ionization: The Intriguing Process of Ion Formation
In the realm of chemistry, the formation of ions is a captivating process known as ionization. Imagine the atomic world where tiny particles dance around, and when they undergo ionization, they transform into charged entities called ions.
Ionization occurs when atoms or molecules acquire an electrical charge by either losing or gaining electrons. This process is driven by the quest for a stable electron configuration, where the number of electrons in an atom’s outermost shell aligns with the rules of chemical bonding.
Types of Ionization
There are two main types of ionization:
- Atomic ionization: When an isolated atom undergoes ionization, resulting in the loss or gain of electrons.
- Molecular ionization: When a molecule undergoes ionization, involving the loss or gain of electrons from the molecule as a whole.
The process of ionization is often influenced by the ionization energy of the atom or molecule. This energy represents the minimum amount of energy required to remove an electron from the outermost shell. Factors like the atomic radius and the number of valence electrons play crucial roles in determining the ionization energy.
Mechanisms of Ionization
Ionization can occur through various mechanisms, including:
- Thermal ionization: Occurs when atoms or molecules are exposed to high temperatures, causing them to release electrons.
- Photoionization: Occurs when atoms or molecules absorb photons of light with sufficient energy to eject electrons.
- Collisional ionization: Occurs when atoms or molecules collide with other charged particles, resulting in the transfer of electrons.
Consequences of Ionization
The formation of ions has profound implications in various scientific fields:
- Chemistry: Ions form the basis of many chemical reactions, including the formation of salts, acids, and bases.
- Biology: Ion channels in cell membranes regulate the transport of substances into and out of cells.
- Physics: Ionizing radiation, such as X-rays and gamma rays, is used in medical imaging and cancer treatment.
Understanding the process of ionization unveils the intricate workings of the atomic world, where tiny particles undergo transformations, shaping the very fabric of our universe.
Valence Electrons and Ion Formation:
- Define valence electrons and explain their role in ion formation.
- Discuss how the number of valence electrons determines an ion’s charge.
- Provide examples to illustrate this concept.
Valence Electrons and the Formation of Ions
In the fascinating world of chemistry, atoms can transform into electrically charged particles called ions. This incredible process, known as ionization, is driven by the behavior of valence electrons, the outermost electrons in an atom’s energy levels.
Valence Electrons: The Key Players
Imagine an atom as a miniature solar system, with protons and neutrons forming the nucleus at the center and electrons, including valence electrons, orbiting around them. Valence electrons play a pivotal role in determining an atom’s chemical properties and its ability to ionize.
Ion Formation: A Balancing Act
When an atom loses or gains an electron, it becomes an ion. The charge of an ion depends on the number of electrons it has gained or lost. Cations are formed when an atom loses one or more valence electrons, resulting in a net positive charge. Conversely, anions are formed when an atom gains one or more valence electrons, acquiring a net negative charge.
Valence Electrons and Ion Charge
The number of valence electrons an atom has directly influences the charge an ion will have. For instance, an atom with two valence electrons can form a cation with a charge of +2 by losing both valence electrons. However, an atom with three valence electrons will form an anion with a charge of -3 if it gains three valence electrons.
Examples of Ion Formation
Let’s explore some specific examples to illustrate this concept:
- Sodium (Na): Sodium has one valence electron. When it loses this electron, it forms a cation with a charge of +1, denoted as Na+.
- Chlorine (Cl): Chlorine has seven valence electrons. When it gains one electron, it forms an anion with a charge of -1, denoted as Cl-.
- Potassium (K): Potassium has one valence electron. When it loses this electron, it forms a cation with a charge of +1, denoted as K+.
- Oxygen (O): Oxygen has six valence electrons. When it gains two electrons, it forms an anion with a charge of -2, denoted as O2-.
Understanding the role of valence electrons in ion formation provides a fundamental insight into the behavior of atoms at the chemical level. It’s a testament to the intricate and fascinating world of chemistry, where the smallest particles can have the most profound impacts.