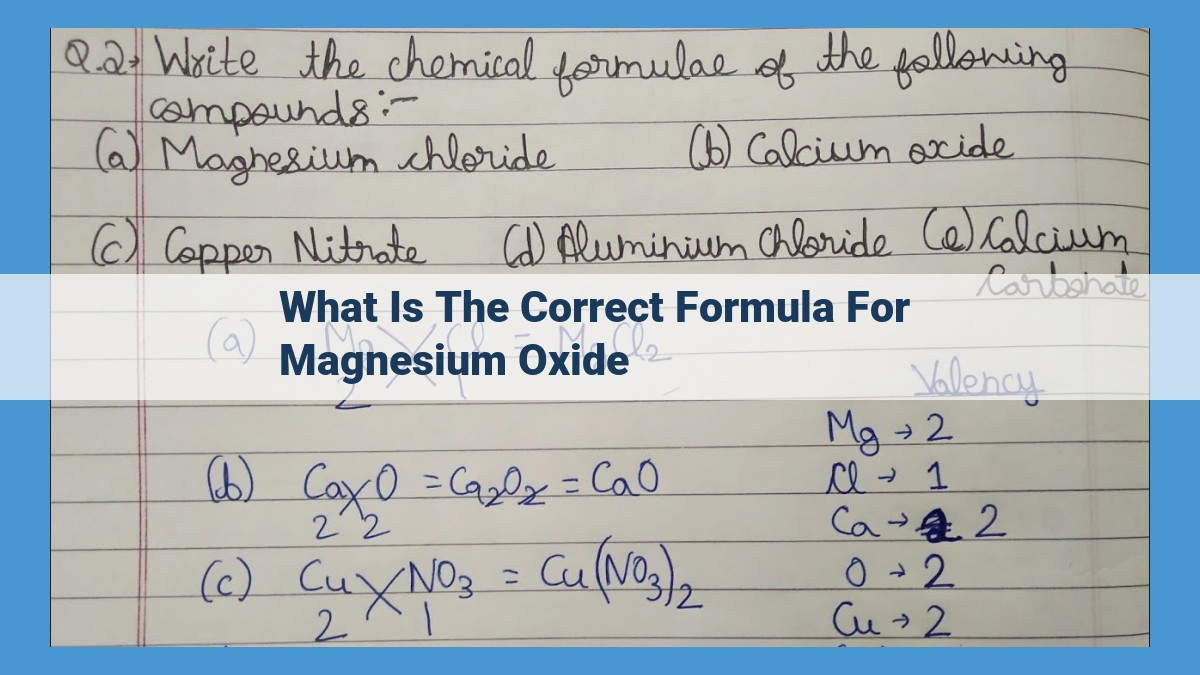
The correct formula for magnesium oxide is MgO. Magnesium oxide is an ionic compound formed when a magnesium cation (Mg2+) and an oxide anion (O2-) combine. The chemical formula of a compound indicates the relative proportions of the elements present in it. In this case, the formula MgO indicates that there is one magnesium atom for every oxygen atom in the compound. The formula also provides information about the charge of the ions involved in the formation of the compound.
Unlocking the Chemical Secrets of Magnesium Oxide: A Story of Ions and Bonding
Magnesium oxide, a white powder with a simple yet intriguing chemical formula, holds a wealth of scientific tales to tell. Its journey begins with understanding the diverse types of chemical formulas, each offering a unique glimpse into the molecular makeup of substances.
Delving into Chemical Formulas
Formulas provide the blueprint for understanding a compound’s composition. Empirical formulas reveal the simplest whole-number ratio of elements in a substance, while molecular formulas pinpoint the exact number of atoms in each molecule.
For magnesium oxide, the empirical formula is MgO, indicating a 1:1 ratio of magnesium to oxygen atoms. Its molecular formula is also MgO, suggesting it exists as a diatomic molecule.
Meet the Players: Cations and Anions
Compounds like magnesium oxide arise from the interplay of ions charged particles. Cations are positively charged ions, like Mg2+ in magnesium oxide, formed by losing electrons. Anions are negatively charged ions, like O2- in magnesium oxide, formed by gaining electrons.
The Ionic Dance: Forming Magnesium Oxide
Magnesium oxide owes its existence to the electrostatic attraction between its oppositely charged ions. When magnesium atoms lose two electrons, they transform into Mg2+ ions. Oxygen atoms, in turn, gain two electrons to become **O2- ions. The strong electrostatic attraction between these ions draws them together, forming a stable ionic compound.
Unveiling the Crystal Structure
The arrangement of ions in crystals reveals their three-dimensional order. Magnesium oxide adopts the NaCl structure (face-centered cubic lattice), where each Mg2+ ion is surrounded by six **O2- ions, and vice versa. This precise arrangement ensures crystal stability and governs the substance’s properties.
Unveiling the Properties of Magnesium Oxide
Magnesium oxide is an electrically insulating material and possesses a high melting point, reflecting its strong ionic bonds. Its thermal stability and chemical inertness make it an invaluable component in refractory materials, construction, and fire retardants.
Magnesium oxide serves as an exemplar of the fascinating world of chemical bonding and ionic compounds. Its simple formula conceals a compelling narrative of electrostatic forces, crystal structures, and properties that underpin its wide-ranging applications. By exploring the chemical formula, we unravel the intricacies of this versatile and enduring material.
Magnesium Cation: The Positively Charged Ion of Magnesium
In the realm of chemistry, cations are positively charged ions formed when an atom or molecule loses one or more electrons. Cations are essential components of ionic compounds, which are formed through the electrostatic attraction between positively charged cations and negatively charged anions.
Among these cations, the magnesium cation (Mg2+) holds a prominent place. It is the positively charged ion of magnesium, a vital element for living organisms. The formation of Mg2+ involves the ionization of a magnesium atom, where an electron is removed from the outermost energy level. This process results in a magnesium cation with a positive charge of 2+, as indicated by the superscript.
Characteristics of the Magnesium Cation (Mg2+):
- Positively charged: Mg2+ carries a double positive charge due to the removal of two electrons.
- Stable electron configuration: After losing two electrons, Mg2+ achieves a stable electron configuration of 1s22s22p6, resembling the noble gas neon.
- Small ionic radius: The loss of electrons reduces the number of energy levels around the magnesium nucleus, resulting in a smaller ionic radius compared to neutral magnesium atoms.
- High polarizing power: The high positive charge of Mg2+ allows it to polarize anions, influencing the distribution of their electron density.
The Oxide Anion: A Deeper Dive
In the realm of chemistry, we encounter fascinating entities known as ions, charged particles that play a crucial role in forming countless compounds. Anions are negatively charged ions, and among them stands out the oxide anion, denoted by the symbol O2-.
The oxide anion is a captivating species, possessing properties that make it a cornerstone of numerous chemical reactions. To understand its significance, let’s delve into its formation and characteristics.
Formation of the Oxide Anion
The oxide anion arises from a process called electron gain. Electronegative atoms, like oxygen, have a strong tendency to attract electrons towards them. When oxygen atoms encounter conditions that favor electron gain, they willingly accept two electrons into their outermost electron shell, transforming into oxide anions.
Characteristics of the Oxide Anion
The oxide anion, O2-, possesses several distinguishing characteristics:
- Charge: It carries a negative charge of two due to the gained electrons.
- Electron Configuration: It has a filled outermost electron shell with eight electrons, making it highly stable and unreactive.
- Size: It is a relatively small ion compared to other anions.
- Polarizability: It is easily polarizable, meaning it can readily distort its electron cloud in the presence of an electric field.
The oxide anion’s properties contribute to its crucial role in forming a vast array of ionic compounds, including the ubiquitous magnesium oxide.
Delving into the Ionic Bond in Magnesium Oxide
In the world of chemistry, the dance of atoms and their interactions forms the cornerstone of matter. Ionic bonds, a special type of chemical bond, are akin to an enchanting waltz between positively and negatively charged ions. Let’s delve into the fascinating story of the ionic bond in magnesium oxide (MgO).
Understanding Ionic Bonds:
Imagine a world where atoms are like tiny magnets, some with a surplus of electrons, making them negatively charged, and others with a shortage, creating a positive charge. These charged particles are called ions. When a positive ion (cation) and a negative ion (anion) meet, an irresistible attraction draws them together like star-crossed lovers. This magnetic bond is what we call an ionic bond.
Formation of Magnesium Oxide:
Our story begins with magnesium, a reactive metal that yearns to shed two electrons, leaving behind a magnesium cation (Mg2+). On the other side of the stage, we have oxygen, a non-metal with a craving for electrons. Oxygen grabs two electrons from magnesium, becoming an oxide anion (O2-).
Like two puzzle pieces, the positively charged Mg2+ and negatively charged O2- ions lock into an electrostatic embrace. This attraction holds them together, creating the ionic compound magnesium oxide (MgO).
The Ionic Waltz:
In the crystal lattice of MgO, the magnesium cations and oxide anions dance in a mesmerizing pattern. They arrange themselves in a face-centered cubic structure, resembling a three-dimensional game of Tetris. Each magnesium ion is surrounded by six oxide ions, and vice versa, forming an intricate lattice of alternating positive and negative charges. This arrangement maximizes the electrostatic attraction, ensuring the stability of the compound.
Properties of Magnesium Oxide:
The ionic character of MgO endows it with unique properties. It boasts a high melting point, low electrical conductivity, and excellent thermal insulation, making it a valuable material in diverse applications such as refractory bricks, construction materials, and electrical insulators.
The ionic bond in magnesium oxide serves as a testament to the captivating interactions between atoms. It showcases the dance between positive and negative ions, resulting in a compound with extraordinary properties that finds use in various fields. By understanding the nature of ionic bonds, we gain a deeper appreciation for the intricate workings of the chemical world around us.
The Lattice Structure of Magnesium Oxide: A Journey into the Atomic Realm
In the world of chemistry, crystals hold a captivating allure, enchanting us with their intricate patterns and fascinating properties. At the heart of every crystal lies its lattice structure, an invisible framework that governs the arrangement of atoms. One such crystal that has captivated scientists for centuries is magnesium oxide (MgO).
Crystal Structure: The Blueprint of Atoms
Imagine a vast, three-dimensional tapestry woven from atoms, meticulously arranged in a repeating pattern. This tapestry is known as the crystal structure. It defines the shape, size, and properties of the crystal as a whole. The arrangement of atoms in a crystal is dictated by two fundamental concepts: the crystal lattice and the space lattice.
The crystal lattice refers to the specific positions of atoms within the crystal. In a space lattice, on the other hand, atoms are arranged at regular intervals, forming a regular geometric pattern. It serves as the underlying framework upon which the crystal lattice is built.
NaCl Structure: The Face-Centered Cubic Lattice of Magnesium Oxide
Magnesium oxide exhibits a face-centered cubic (FCC) lattice structure, also known as the NaCl structure. In this arrangement, magnesium ions (Mg2+) occupy the corners and oxide ions (O2-) reside in the center of each face of the cube. This precise arrangement gives rise to the crystal’s unique properties.
The FCC lattice is characterized by its high coordination number, meaning that each magnesium ion is surrounded by six oxide ions and vice versa. This intimate arrangement of ions results in strong electrostatic forces, contributing to the crystal’s exceptionally high melting point and hardness.
Applications: Unlocking the Potential of Magnesium Oxide
The remarkable properties of magnesium oxide have earned it a wide range of applications in various industries. Its high melting point and electrical insulation make it an ideal refractory material, used in the linings of furnaces and crucibles. Its ionic character endows it with antibacterial and antifungal properties, making it a valuable additive in medical products.
Moreover, magnesium oxide’s low thermal conductivity and high refractive index render it suitable for use in optics and electronics. Its abrasive properties are also harnessed in polishing powders and grinding materials.
In the realm of chemistry, understanding the lattice structure of magnesium oxide provides a deeper insight into its behavior and properties. This knowledge empowers scientists and engineers to tailor materials with specific characteristics, unlocking endless possibilities for technological advancements.
Unveiling the Properties and Applications of Magnesium Oxide
Magnesium oxide, an intriguing chemical compound, possesses exceptional properties that make it indispensable in various industries. Its high melting point, ionic character, and electrical insulation are the cornerstones of its versatility.
High Melting Point: Resilience in the Face of Heat
Magnesium oxide stands out with its exceptionally high melting point of 2,852 °C. This remarkable attribute stems from the strong electrostatic attraction between the magnesium cation (Mg2+) and the oxide anion (O2-). The intense ionic bonding results in a tightly bound crystal lattice that requires an enormous amount of energy to break apart and hence, a stratospheric melting point.
Ionic Character: The Foundation of a Stable Crystal
Magnesium oxide exemplifies the essence of an ionic compound. The transfer of electrons from magnesium to oxygen creates a disparity in charge, transforming them into cations and anions, respectively. These oppositely charged ions are then held together by electrostatic forces, forming a stable crystal structure. The ionic nature of magnesium oxide underscores its high melting point and low electrical conductivity, as the ions cannot move freely to conduct electricity.
Electrical Insulation: A Guardian Against Electric Flow
As an electrical insulator, magnesium oxide exhibits a formidable resistance to the passage of electric current. This property stems from its tightly bound ionic structure, which prevents the movement of electrons. Magnesium oxide is a cornerstone in electrical applications, effectively preventing unwanted current flow and short circuits.
Applications: Harnessing Magnesium Oxide’s Extraordinary Attributes
The remarkable properties of magnesium oxide have propelled it to the forefront of numerous applications.
-
Refractory Materials: Magnesium oxide’s high melting point and thermal stability make it a prime candidate for refractory materials in furnaces and kilns.
-
Insulating Materials: Its electrical insulation prowess renders magnesium oxide ideal for electrical insulators and high-voltage applications.
-
Medical Applications: The biocompatibility and low toxicity of magnesium oxide find use in bone cement, dental implants, and drug delivery systems.
Magnesium oxide, a seemingly unassuming chemical compound, conceals a wealth of extraordinary properties. Its high melting point, ionic character, and electrical insulation have catapulted it to the forefront of diverse industrial applications, making it an indispensable material in modern society. Understanding these properties empowers us to harness the full potential of this remarkable substance, unlocking the door to further advancements and innovations.