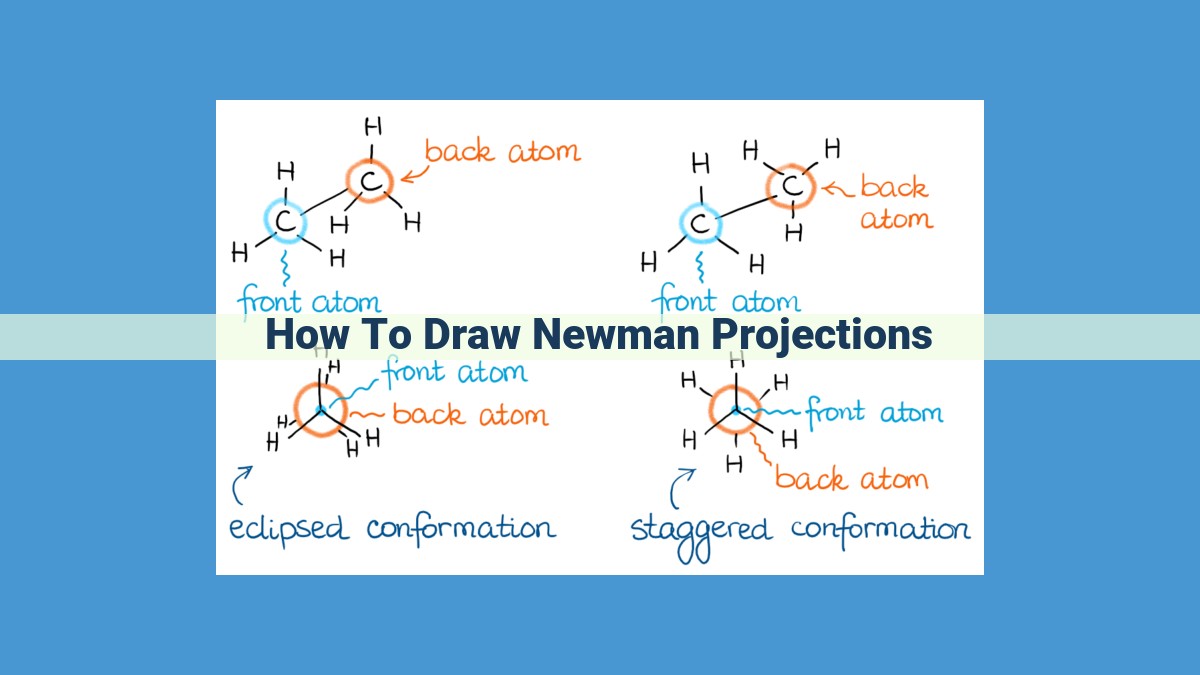
Newman projections are a method of visualizing the spatial arrangement of atoms in a molecule. They represent carbon atoms as circles and bonds as lines, with the front carbon atom shown larger. The projection allows us to view the molecule along a specific bond, helping us understand its conformation and potential energy differences. To draw a Newman projection, we first determine the front and rear carbon atoms, then arrange the remaining atoms around them. By understanding the principles of Newman projections, we can predict molecular shape, analyze conformational isomers, and explore advanced concepts such as rotational barriers and conformational analysis.
Newman Projections: A Guide to Visualizing Molecular Structure
In the realm of chemistry, understanding the spatial arrangement of molecules is crucial. Newman projections emerge as a powerful tool for visualizing this three-dimensional architecture, aiding us in comprehending the shape and behavior of organic molecules.
Newman projections project a molecule onto a flat plane, with the carbon backbone represented as a circle. The bonds extending from the carbon atoms are portrayed as lines, providing a simplified but insightful perspective of the molecule’s molecular structure.
Delving into Basic Principles
Newman projections adhere to a set of fundamental principles. The circles symbolize carbon atoms, while lines depict bonds. The carbon atoms in the foreground and background are differentiated, creating a deeper understanding of the molecule’s orientation.
Common Conformations and Their Energetics
Molecules can adopt distinct conformations, each characterized by its unique arrangement of atoms. Common conformations include staggered, eclipsed, gauche, and anti. Their potential energies vary significantly, with the eclipsed and staggered conformations representing the highest and lowest energy states, respectively.
Step-by-Step Guide to Drawing Newman Projections
Drawing Newman projections involves a step-by-step approach:
- Identify the central carbon atom: Choose the carbon atom of interest as the focus of the projection.
- Represent the bonds as lines: Draw lines from the central carbon atom to its bonded atoms.
- Determine the front and rear carbon atoms: Distinguish between the carbon atoms in front and behind the projection plane.
- Position the substituents: Place the substituents (atoms or groups) on the front and rear carbon atoms accordingly.
Basic Principles of Newman Projections
In the world of molecular structures, understanding the arrangement of atoms and bonds is key. Newman projections offer a graphical tool for visualizing these structures, providing a simplified and intuitive perspective.
At the heart of Newman projections lies the representation of carbon atoms as circles. These circles symbolize the carbon atom itself, while the bonds connecting them are sketched as lines radiating from the circles. This simple representation allows us to focus on the spatial relationships between the atoms, rather than getting bogged down in complex three-dimensional drawings.
Another crucial concept in Newman projections is the distinction between front and rear carbon atoms. The circle closest to the observer represents the front carbon, while the one behind it is the rear carbon. This distinction is essential for understanding the orientation and interactions between the various bonds and atoms in the molecule.
Mastering these basic principles is the foundation for effectively using Newman projections to comprehend and predict molecular structures, paving the way for further exploration into their applications and advanced concepts.
Common Conformations and Their Energies
When it comes to the arrangement of atoms in a molecule, conformations play a crucial role. Conformations refer to the different ways in which atoms can be oriented around specific bonds. In this context, we’re focusing on Newman projections, which provide a simplified representation of molecular structure.
Imagine a carbon-carbon bond as a line connecting two circles representing the carbon atoms. This is the foundation of Newman projections. In the staggered conformation, the bonds to the front carbon atom are pointing in opposite directions to the bonds on the rear carbon atom. This arrangement minimizes steric hindrance, or the repulsive interactions between electron clouds of nearby atoms, and results in the lowest potential energy.
Moving on to the eclipsed conformation, we find the bonds on the front and rear carbon atoms aligned directly opposite each other. This orientation maximizes steric hindrance, leading to a higher potential energy.
In between these two extremes lies the gauche conformation, where the bonds on the front and rear carbon atoms are staggered by 60 degrees. This conformation has a higher potential energy than the staggered conformation but lower than the eclipsed conformation.
Lastly, the anti conformation is a special case where the bonds on the front and rear carbon atoms are aligned 180 degrees apart. This conformation is equivalent in energy to the staggered conformation.
To summarize, the potential energy of a conformation depends on steric hindrance. The staggered conformation, with minimal steric hindrance, has the lowest potential energy, followed by the gauche conformation, the eclipsed conformation, and the anti conformation. Understanding these conformations and their energies is essential for predicting molecular shape and analyzing conformational isomers.
Drawing Newman Projections Step-by-Step: A Beginner’s Guide
Mastering the art of drawing Newman projections can unlock a deeper understanding of molecular structure and behavior. Here’s a step-by-step guide to help you visualize and depict these essential chemical representations:
Step 1: Identify the Central Carbon
Choose the carbon atom you wish to represent as the front carbon and the one behind it as the rear carbon. These carbons will be depicted as circles.
Step 2: Draw the Front Carbon
Draw a circle representing the front carbon. Remember, you’re looking directly at it, so you’re not seeing any bonds behind it.
Step 3: Draw the Rear Carbon
Behind the front carbon, draw another circle representing the rear carbon. Connect the two circles with a line to represent the bond between them.
Step 4: Add Substituents
Identify any substituent atoms or groups attached to the front and rear carbons and draw them as lines extending from the respective circles. For example, hydrogen atoms are represented by single lines.
Step 5: Connect the Substituents
Connect the substituent lines to the appropriate circle, ensuring that each bond between a carbon and a substituent is represented by a single line.
Step 6: Rotate the Projection
If necessary, rotate the Newman projection to clearly show any steric interactions or other features of interest. For instance, you can rotate to view a staggered or eclipsed conformation.
Example:
Let’s draw the Newman projection of ethane (CH3CH3):
- Front carbon: Circle with three lines (H-C-H)
- Rear carbon: Circle with three lines (H-C-H)
- Bond: Line connecting the circles
- Substituents: None
The resulting Newman projection is a staggered conformation, where the hydrogen atoms on the front and rear carbons are as far apart as possible.
Applications of Newman Projections
Newman projections, a powerful tool in structural chemistry, offer a simplified way to visualize and understand the three-dimensional arrangement of atoms in molecules. Their applications extend beyond mere visualization, expanding into realms of predicting molecular shape and analyzing conformational isomers.
Molecular Shape Prediction
Newman projections provide a clear picture of the relative positions of different groups attached to a carbon atom. By analyzing the spatial arrangement of these groups, it becomes possible to predict the overall shape of the molecule. For instance, in ethane, the staggered conformation, where hydrogen atoms are positioned away from each other, results in a staggered molecular shape.
Conformational Isomer Analysis
Newman projections shine when it comes to analyzing conformational isomers. These are molecules with the same molecular formula but different spatial arrangements of atoms. By constructing Newman projections for each isomer, chemists can compare their energies and determine which one is the most stable.
In the case of butane, there are three different conformational isomers: anti, gauche, and eclipsed. The anti isomer is the most stable because its methyl groups are positioned as far apart as possible, minimizing steric hindrance. Conversely, the eclipsed isomer is the least stable due to its close proximity of methyl groups.
By harnessing the insights provided by Newman projections, chemists can gain a deeper understanding of molecular structure and its impact on molecular behavior. This knowledge forms the foundation for drug design, materials science, and other fields where an intricate comprehension of molecular properties is paramount.
Advanced Concepts
- Discuss the role of alkyl groups and bond lengths in Newman projections.
- Introduce the concept of rotational barriers and conformational analysis.
Advanced Concepts in Newman Projections
As we delve deeper into the realm of Newman projections, we encounter more intricate concepts that shed light on the nuances of molecular structure and behavior. Two key aspects to consider are the role of alkyl groups and bond lengths, as well as the concept of rotational barriers and conformational analysis.
Influence of Alkyl Groups and Bond Lengths
Newman projections reveal not only the arrangement of carbon atoms, but also the substituents attached to them. Alkyl groups, consisting of carbon and hydrogen atoms, when attached to a carbon atom, influence its conformation. Larger alkyl groups create more steric hindrance, making certain conformations less stable. Additionally, bond lengths between carbon atoms and substituents can impact the exact geometry of the molecule.
Rotational Barriers and Conformational Analysis
Newman projections provide a framework for understanding the energy barriers encountered when rotating around single bonds. As a molecule rotates, it passes through different conformations, each with its unique energy. The most stable conformation is the one with the lowest energy. The energy difference between conformations is known as the rotational barrier. Conformational analysis involves determining the stable conformations and their relative energies. By understanding these concepts, we gain deeper insights into the conformational behavior of molecules.