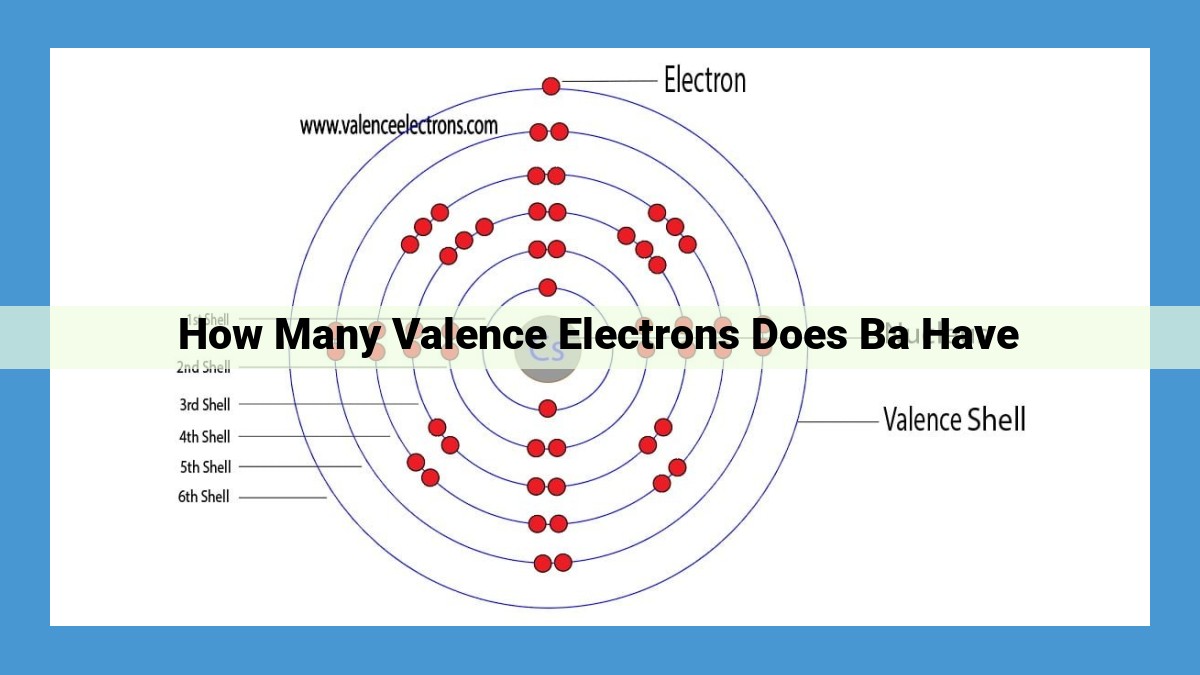
- Valence electrons, found in the outermost shell, determine an element’s chemical reactivity. Barium, an alkaline earth metal, has 2 valence electrons, as indicated by its atomic number of 56. The periodic table reveals this information, showing barium’s location in Group 2 and Period 6, aligning with the trend of increasing valence electrons within each group.
Valence Electrons: A Key Concept
- Definition and characteristics of valence electrons
- Their role in forming chemical bonds
Valence Electrons: The Building Blocks of Chemical Bonds
In the captivating world of chemistry, valence electrons, like tiny magnets, hold the power to unite atoms and create the rich tapestry of molecules that surround us. These electrons, residing in the outermost energy levels of an atom, are the key players in the dance of chemical bonding.
Definition and Characteristics of Valence Electrons
Valence electrons are like the social butterflies of the atomic world. They are the most energetic and loosely held electrons, eager to mingle with others to form connections. The number of valence electrons an atom possesses varies depending on its atomic number, which is the unique identifier for each element.
Role in Forming Chemical Bonds
Valence electrons are the matchmakers of the atomic realm. They determine an atom’s ability to bond with others, forming the building blocks of molecules and compounds. This bonding behavior is driven by the irresistible desire of atoms to achieve a stable electron configuration, typically with eight valence electrons.
Examples
Let’s delve into some examples. Sodium, with just one valence electron, readily gives it up to achieve a stable configuration. Chlorine, on the other hand, has seven valence electrons and eagerly accepts one to complete its electron arrangement. When these two atoms encounter each other, a magical dance occurs. Sodium donates its lone electron, while chlorine accepts it, resulting in the formation of a sodium chloride molecule.
Importance in Understanding Chemical Bonding
By grasping the concept of valence electrons, we unlock a deeper understanding of the intricate world of chemical bonding. It allows us to predict the reactivity of elements, unravel the mysteries of molecular structures, and delve into the fascinating realm of chemical processes that shape our world.
Exploring the Fascinating Element: Barium
In the realm of chemistry, where elements dance in a harmonious symphony, there exists a captivating element known as barium. This alkaline earth metal holds a special place in the periodic table, its story intertwining with concepts of atomic structure and chemical bonding.
Barium resides within Group 2, where all elements share a common trait: two valence electrons. These outermost electrons are the key players in the element’s chemical interactions, making it highly reactive and eager to form bonds with other atoms.
Barium’s position on the periodic table also reveals its atomic number, a unique identifier for each element. With 56 protons in its nucleus, barium possesses an equal number of electrons, giving it an overall neutral charge. This atomic number directly influences barium’s electron configuration, the arrangement of its electrons within orbitals.
Electrons, like miniature planets, orbit the nucleus in specific energy levels, known as shells. Barium’s electron configuration, [Xe] 6s², reveals that it has a full outermost shell, containing two electrons. These valence electrons occupy the 6s orbital and are crucial for understanding barium’s chemical behavior.
In the captivating tale of chemical bonding, barium’s valence electrons take center stage. These electrons become the key participants in forming bonds with other elements, holding atoms together like dancers in a graceful embrace. Barium’s strong attraction to oxygen, for example, is due to its desire to complete its valence shell by gaining two electrons, forming stable ionic bonds with oxygen.
Barium’s journey through the world of chemistry is filled with fascinating discoveries and insights. Its unique atomic structure and electron configuration make it an element of great importance, both in the natural world and in the field of scientific research.
The Power of the Periodic Table: Unraveling the Secrets of Chemistry
The periodic table is a masterpiece of scientific organization, an arrangement of elements that unveils the profound secrets of chemistry. It’s a table that speaks volumes about the structure and properties of matter, guiding us through the intricate tapestry of the chemical world.
Within its ordered rows and columns, the periodic table reveals the fundamental principles that govern the behavior of elements. Periodic trends, gradual changes in properties as we move across or down the table, provide invaluable insights into atomic structure and bonding.
For instance, the metals reside on the left side of the table, characterized by their gleaming, lustrous appearance and ability to conduct electricity. As we progress to the right, we encounter the nonmetals, which possess the opposite traits: dull, brittle, and poor conductors. This trend directly relates to the valence electrons of these elements—the electrons in their outermost energy level that dictate their chemical reactivity.
The periodic table also empowers us to predict chemical behavior. By examining an element’s position, we can infer its:
- Reactivity: Highly reactive elements tend to lie in the top left of the table, while the least reactive reside in the bottom right.
- Bonding tendencies: Elements in the same group (vertical column) generally share similar bonding characteristics.
- Oxidation state: The group number often indicates the common oxidation states of an element.
The periodic table is not merely a static map but a dynamic tool for chemical exploration. It serves as a guide, revealing the connections between elements, their properties, and reactivities. By harnessing the power of the periodic table, scientists and chemists unlock the secrets of matter, from the simplest atoms to the most complex compounds.
Atomic Number: The Cornerstone of Element Identity
In the vast tapestry of chemistry, elements stand as the building blocks of the natural world. Each element possesses a unique set of characteristics that define its behavior and distinguish it from all others. Among these characteristics, the atomic number reigns supreme as the fundamental identifier of an element’s very essence.
The atomic number, often denoted by the symbol Z, represents the number of protons in an atom’s nucleus. This number serves as the central pillar of an element’s identity, as it dictates the number of electrons that orbit the nucleus. The balance between protons and electrons determines an element’s valence, its ability to form chemical bonds with other atoms.
Furthermore, the atomic number establishes an element’s position within the periodic table. Elements are arranged in the periodic table according to their atomic numbers, which increase from left to right and from top to bottom. This arrangement reveals periodic trends in chemical properties, such as reactivity and valence. For instance, elements in the same column of the periodic table (also known as a group) typically exhibit similar chemical behavior due to their shared number of valence electrons.
Therefore, the atomic number not only provides a unique identifier for each element but also serves as a guidepost for understanding its chemical properties and its place in the broader framework of the periodic table. It is the cornerstone upon which our understanding of the elements and their interactions is built.
Unveiling Electron Configuration
- Distribution of electrons in atomic orbitals
- Techniques used to determine electron configuration
- Importance in understanding chemical bonding
Unveiling Electron Configuration: A Journey into the Quantum Realm
Distribution of Electrons in Atomic Orbitals
Like celestial bodies orbiting the nucleus, electrons dance within an atom in discrete energy levels called orbitals. Each orbital can hold a specific number of electrons, with the first orbital (closest to the nucleus) holding two while subsequent orbitals hold eight. These orbitals are arranged in three-dimensional space, appearing like spherical clouds or dumbbells.
Techniques to Determine Electron Configuration
Scientists employ various techniques to unravel the distribution of electrons in atoms. Spectroscopy, like a cosmic telescope, examines the energy released or absorbed by atoms as electrons transition between orbitals. Quantum mechanics, the mathematical framework of the atomic realm, also guides the determination of electron configuration.
Importance for Chemical Bonding
The electron configuration of an atom holds the key to its chemical behavior. It governs the number and type of bonds an atom can form. Elements with similar electron configurations exhibit similar chemical properties. For instance, alkali metals, with a single valence electron, readily react to form ionic bonds.
Electron configuration is a fundamental concept in chemistry, unveiling the arrangement of electrons within atoms. It serves as a guide to predict chemical bonding and understand the remarkable diversity of elements in our universe. As we delve deeper into the electron configuration of elements, we gain invaluable insights into the intricate tapestry of the microscopic world.