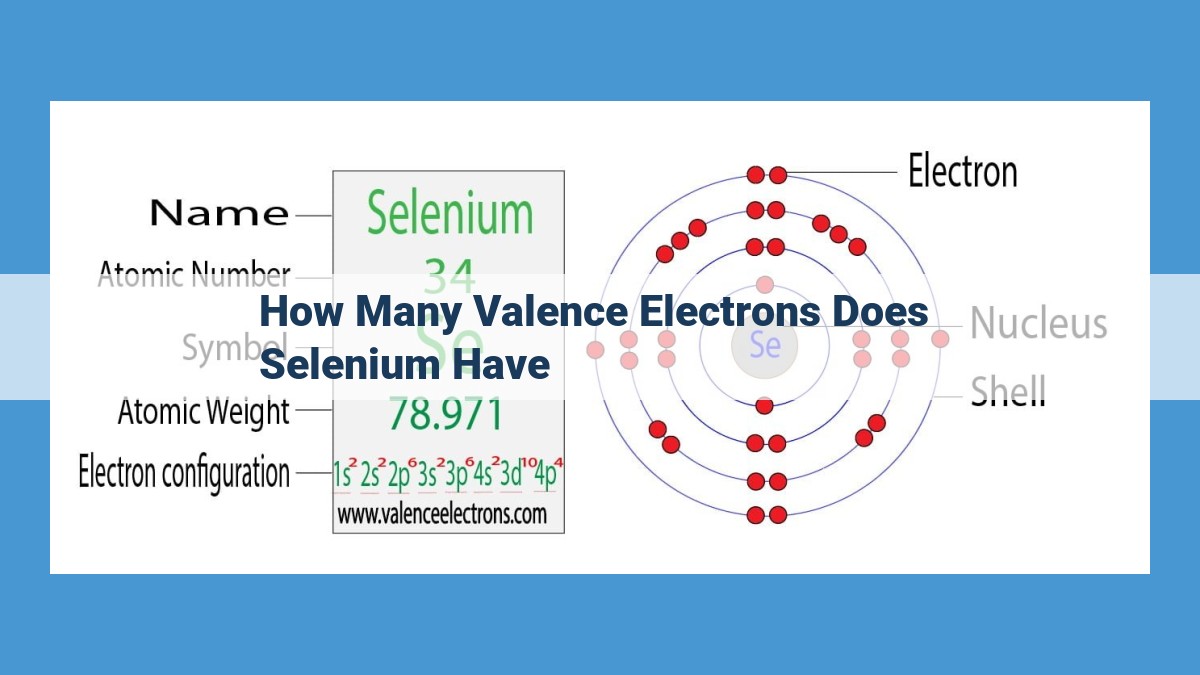
Selenium, located in Group 16 of the Periodic Table, has an atomic number of 34. Determining its valence electrons involves understanding the concept of valence electrons, defined as the outermost electrons involved in chemical bonding. By referring to the Periodic Table, we can observe that elements in Group 16 typically have six valence electrons. Therefore, Selenium has six valence electrons, which play a crucial role in its chemical properties and bonding behavior.
Unveiling the Essence of Valence Electrons: A Journey into Chemical Bonding
In the realm of chemistry, understanding the concept of valence electrons is pivotal in comprehending the behavior and interactions of elements. Valence electrons, like miniature engineers of the atomic world, reside in the outermost shell of an atom and play a crucial role in determining its chemical properties and bonding capabilities.
These electrons are the key players in the fascinating process of chemical bonding, the glue that holds atoms together to form molecules and compounds. Imagine them as the social butterflies of the atomic world, eager to interact and form connections with other electrons. By understanding valence electrons and their significance, we embark on a journey into the captivating world of chemical bonding.
The Role of Atomic Number in Understanding Valence Electrons
In the realm of chemistry, understanding the behavior of elements is crucial for unraveling the mysteries of chemical reactions. One fundamental aspect of this understanding lies in comprehending the concept of valence electrons, which are the outermost electrons in an atom that actively participate in chemical bonding. The number of valence electrons an element possesses can profoundly influence its reactivity and determine its position in the Periodic Table.
At the core of this fascinating story lies atomic number, a defining characteristic that distinguishes one element from another. Atomic number represents the number of protons residing in an atom’s nucleus. These positively charged particles not only contribute to the element’s identity but also orchestrate the arrangement of electrons within the atom’s electron cloud. The number of protons within an atom dictates the number of electrons it will acquire to achieve a neutral state.
For instance, let’s consider the element sodium, renowned for its energetic reactions. Sodium’s atomic number is 11, signifying the presence of 11 protons in its nucleus. Consequently, sodium will attract 11 electrons to balance its positive charge and attain a neutral state. Crucially, the outermost electron in sodium‘s electron cloud, the valence electron, plays a pivotal role in its chemical interactions. The number of valence electrons present in an element often aligns with its atomic number. This relationship provides a valuable clue in determining the valence electron count of an element.
The Periodic Table, a systematic arrangement of elements, ingeniously organizes elements based on their atomic numbers and properties. This tabular masterpiece serves as an invaluable tool for predicting the valence electron count of elements. Each element occupies a specific position within the Periodic Table, dictated by its atomic number. By carefully examining an element’s location in the Periodic Table, scientists can deduce its valence electron count with remarkable accuracy.
In conclusion, atomic number stands as a fundamental property that shapes the identity of an element, governs the number of electrons it acquires, and ultimately influences the element’s valence electron count. This intricate relationship between atomic number and _valence electrons unlocks a deeper understanding of chemical bonding and paves the way for unraveling the captivating world of chemistry.
Exploring the Periodic Table
- Discuss the organization and structure of the Periodic Table, including the arrangement of elements based on atomic number and properties.
Exploring the Periodic Table: A Journey into Element Organization
The Periodic Table, a masterpiece of scientific classification, serves as a map that unveils the secrets of the elements. Arranged in ascending order of atomic number, the elements unfold their enigmatic properties, revealing insights into their fundamental nature.
Each element occupies a designated spot on this table, its position meticulously determined by its atomic number. This enigmatic number signifies the number of protons residing within the nucleus, the very core of an atom. By understanding the intricacies of atomic number, we unlock the key to element identification and unravel the mysteries of their unique personalities.
The Periodic Table showcases elements grouped into distinct blocks. Like pieces in a puzzle, these blocks categorize elements based on the number and arrangement of their valence electrons. Valence electrons, the outermost electrons in an atom, dictate the element’s chemical reactivity, determining its willingness to forge bonds with other elements.
As we traverse this table, we encounter elements like noble gases – inert observers that remain aloof from chemical unions due to their stable electron configurations. In contrast, other elements, such as alkali metals, eagerly surrender their solitary valence electron to form ionic bonds, creating the foundation for countless compounds.
The Periodic Table, with its vertical columns (groups) and horizontal rows (periods), provides a treasure-trove of information about each element. By delving into its depths, we discover patterns and relationships, enabling us to predict the properties of elements and comprehend their behavior in the world around us.
Selenium: Unveiling its Valence Electrons
In the world of chemistry, understanding the concepts of valence electrons is crucial in unraveling the behavior of elements. Valence electrons are the electrons orbiting an atom’s outermost energy level, and they play a pivotal role in chemical bonding.
Now, let’s take a closer look at selenium, an element with unique properties and an intriguing number of valence electrons.
Selenium’s Place in the Periodic Table
Selenium resides in the Group 16 of the Periodic Table, also known as the chalcogens. Elements within this group share similar properties, including a characteristic valence electron count of six. This means that selenium possesses six valence electrons in its outermost energy level.
Determining Valence Electrons: A Three-Step Process
Understanding the concepts of valence electrons, atomic number, and Periodic Table properties is fundamental in determining the valence electron count of any element. For selenium:
- Atomic Number: Selenium’s atomic number is 34, indicating the presence of 34 protons in its nucleus.
- Periodic Table Properties: As mentioned earlier, elements in Group 16 have six valence electrons.
- Therefore: Selenium, being in Group 16, also has six valence electrons.
Implications of Selenium’s Valence Electrons
Selenium’s six valence electrons dictate its chemical behavior. These electrons actively participate in chemical bonding, enabling selenium to form covalent bonds with other atoms. In elemental form, selenium exists as a polyatomic chain due to the covalent bonds between its atoms.
Furthermore, selenium’s valence electrons influence its oxidative properties. It can exist in various oxidation states, ranging from -2 to +6. This versatility allows selenium to react with a wide range of elements, contributing to its involvement in numerous chemical reactions and applications.
Understanding the valence electrons of an element, like selenium, is vital in predicting its chemical behavior. Selenium’s six valence electrons make it a reactive element capable of forming covalent bonds and exhibiting diverse oxidation states. By unraveling the secrets of valence electrons, we gain valuable insights into the world of chemistry and the elements that shape it.
Determining Selenium’s Valence Electrons: A Step-by-Step Guide
To determine selenium’s valence electrons, let’s delve into the intriguing world of atomic structure and the Periodic Table.
1. Understanding Atomic Number:
- Every element in the Periodic Table has a unique atomic number, which represents the number of protons in its nucleus.
- The atomic number determines the element’s identity and its position on the Periodic Table.
2. Relating Atomic Number to Valence Electrons:
- The valence electrons are the electrons in the outermost energy level of an atom, responsible for chemical bonding.
- The atomic number can help predict the number of valence electrons. For instance, selenium has an atomic number of 34, indicating 34 protons in its nucleus.
3. Exploring the Periodic Table’s Clues:
- The Periodic Table organizes elements in a way that highlights their properties.
- Selenium is located in Group 16 (also known as the Oxygen group) of the Periodic Table.
- Elements within the same group share similar chemical characteristics, including the number of valence electrons.
4. Applying the Periodic Table Insights:
- Group 16 elements, like selenium, tend to have six valence electrons. This is because Group 16 lies to the right of the noble gases, which have a stable octet of electrons in their outermost energy level.
- Selenium aims to achieve this stable configuration by gaining or sharing six valence electrons.
Therefore, by combining our understanding of atomic number, the Periodic Table’s organization, and the concepts of valence electrons, we can confidently determine that selenium has six valence electrons. This knowledge paves the way for comprehending selenium’s chemical behavior and reactivity.