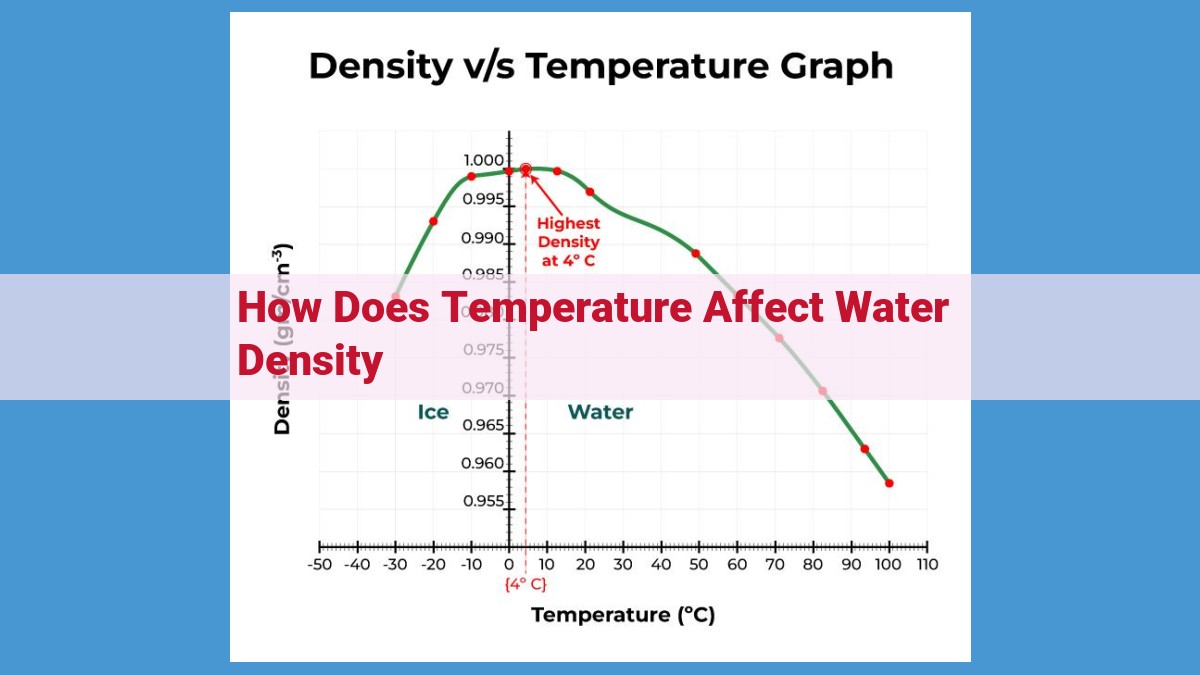
Understanding the temperature-water density relationship is vital. Temperature inversely affects water density due to hydrogen bonding. At higher temperatures, hydrogen bonds weaken, leading to decreased molecular packing and reduced water density. This relationship is significant in various fields, including oceanography, meteorology, and industrial processes.
The Curious Case of Water Density and Temperature: A Hydrological Tale
As we dive into the realm of our planet’s lifeblood, water, we encounter a fascinating phenomenon: temperature plays a pivotal role in shaping its density. Water density, the measure of its mass per unit volume, is a key factor in understanding its behavior in different environments.
The Inverse Relationship: A Tale of Temperature and Density
Unlike most substances, water behaves in an unusual way when it comes to temperature and density. As the temperature increases, the density of water decreases. This inverse relationship is evident in our everyday experiences. Think of a warm cup of tea compared to a cold glass of water. The warm tea is less dense and floats atop the colder water.
The Mystery Unraveled: Hydrogen Bonding’s Role
The secret behind this paradoxical behavior lies in the hydrogen bonding within water molecules. Hydrogen bonding is a delicate dance between molecules, where a hydrogen atom from one molecule is attracted to an electronegative atom (usually oxygen) in another. In water, these bonds create a intricate web that holds the molecules closely together.
Heat Disrupts the Molecular Dance
As temperature rises, the energy of the water molecules increases. This extra energy weakens the hydrogen bonds, causing a disruption in the molecular packing. Consequently, the water molecules become less tightly bound, leading to a decrease in water density.
Temperature: The Catalyst for Molecular Unrest
Temperature is the driving force behind this molecular disruption. Higher temperatures provide more energy to the molecules, enabling them to overcome the attractive forces of hydrogen bonding. As a result, the molecules become less densely packed, causing the overall density of water to decrease.
Significance and Applications
Understanding the temperature-water density relationship has profound implications. It helps us comprehend phenomena like the formation of ice on water’s surface, where less dense warm water floats above denser cold water. This knowledge finds practical applications in various fields, including oceanography, engineering, and food processing.
Water Density and Temperature
- Inverse relationship between temperature and water density
How Temperature Shapes Water’s Density and Significance
Water’s Essence: Understanding Density
Water, the elixir of life, is a crucial component of our planet and our bodies. Its unique properties play a vital role in countless processes, and among them, density holds a special significance. Density, a measure of how tightly packed matter is, helps water shape our world in remarkable ways.
The Inverse Dance of Temperature and Density
When it comes to water, temperature orchestrates a curious dance with density. As water warms, something peculiar happens: its density decreases. This phenomenon is contrary to what we might expect from most other substances. Solids and gases typically become less dense as they heat up, but not so with water.
The Role of Hydrogen Bonds: A Molecular Embrace
To unravel the mystery behind this inverse relationship, we must delve into the molecular realm. Water molecules form hydrogen bonds, weak attractions between hydrogen and oxygen atoms. These bonds create a loosely woven network, holding water molecules together.
The Thermal Disruption: Hydrogen Bonds Under Fire
As water heats up, the molecules gain energy, and their frantic dance intensifies. This increased energy disrupts the delicate hydrogen bonds, weakening them and causing them to break. Consequently, the water molecules can pack less efficiently, resulting in a decrease in density.
The Significance of Temperature: Energy and Bond Strength
Temperature is a measure of the average energy of the molecules in a substance. The higher the temperature, the greater the molecular energy. This increased energy leads to a higher disruption of hydrogen bonds and a subsequent decrease in water density.
Understanding the inverse relationship between temperature and water density is not just an academic curiosity; it has practical implications in various fields. From ocean currents to the regulation of biological systems, this unique property of water shapes the world around us. By appreciating the role of hydrogen bonds and temperature, we gain a deeper understanding of the fundamental nature of water, a precious resource that sustains life on Earth.
Hydrogen Bonding: The Silent Architect of Water’s Density
In the realm of water, temperature reigns supreme as a master manipulator, dictating the liquid’s density. This intricate dance between temperature and density is orchestrated by a hidden force known as hydrogen bonding.
Hydrogen bonds are like tiny bridges, connecting water molecules. They arise when a hydrogen atom, covalently bonded to one oxygen atom, forms a weak bond with an adjacent oxygen atom. This creates an electrostatic attraction between the molecules, holding them together like a molecular web.
As temperature rises, these hydrogen bonds come under siege. The kinetic energy of the water molecules increases, causing them to move more vigorously. This jostling weakens the hydrogen bonds, disrupting the tightly packed molecular structure of water.
With the hydrogen bonds compromised, the molecules become more separated. This decrease in molecular packing reduces the density of water. Simply put, warmer water becomes less dense than its cooler counterpart.
Understanding the Inverse Relationship Between Temperature and Water Density
Water, the elixir of life, is a remarkable substance whose properties are intricately influenced by temperature. Among these properties, density stands out as a fundamental characteristic that plays a crucial role in various natural phenomena and industrial applications.
The density of water, a measure of its mass per unit volume, is inversely related to temperature. As temperature rises, water density decreases, and vice versa. This intriguing relationship is primarily attributed to the unique hydrogen bonding that exists between water molecules.
Hydrogen bonds are intermolecular forces that arise from the electrostatic attraction between the slightly positively charged hydrogen atoms of one water molecule and the slightly negatively charged oxygen atom of another. These bonds form a three-dimensional network that holds water molecules together, giving it its liquid state.
When temperature increases, the kinetic energy of water molecules also increases. This increased energy causes the molecules to move faster and more erratically, disrupting the hydrogen bonds that hold them together. As the hydrogen bonds weaken, the water molecules become less tightly packed, resulting in a decrease in density.
Imagine a crowd of people standing close together. As the temperature rises, the people start to move around more vigorously, bumping into each other and disrupting the tight formation. This increased movement corresponds to the increased kinetic energy of water molecules at higher temperatures, leading to a “loosening” of the molecular structure and a decrease in density.
This inverse relationship between temperature and water density has profound implications in various fields. For instance, in oceanography, it influences the circulation patterns of ocean currents, which are driven by density differences. In limnology, it affects the thermal stratification of lakes, where warmer, less dense water tends to float on top of colder, denser water.
Understanding the temperature-water density relationship is essential for a wide range of applications, including the design of pipelines, cooling systems, and power plants that rely on water as a coolant. By appreciating the role of hydrogen bonding in this relationship, we can harness the unique properties of water to optimize their performance and efficiency.
Temperature: The Invisible Force Manipulating Water’s Density
Temperature, an imperceptible yet profound force, exerts a significant influence on the density of water, a substance so essential to life on Earth. Understanding this relationship is crucial for unraveling the mysteries of our planet’s oceans, lakes, and rivers.
Defining Temperature
Temperature represents the degree of heat within a system, indicating the kinetic energy of its molecules. This energy manifests as random motion, with higher temperatures corresponding to faster molecular movement.
Temperature’s Correlation to Molecular Energy
The relationship between temperature and molecular energy is direct and proportional. As temperature increases, so does the molecular energy. This heightened energy disrupts the hydrogen bonds that hold water molecules together.
Weakening Hydrogen Bonds
Hydrogen bonds are intermolecular forces that arise between water molecules due to their unique structure. These bonds create a network that stabilizes water, contributing to its high density. However, with increasing temperature, these hydrogen bonds weaken.
As molecular energy increases, the kinetic energy of water molecules surpasses the strength of hydrogen bonds. This results in the disruption of these bonds, allowing water molecules to move more freely. Consequently, the molecular packing decreases, leading to a reduction in density.
In essence, temperature acts as an invisible puppeteer, controlling the strength of hydrogen bonds and, subsequently, the density of water. Comprehending this relationship is vital for understanding various phenomena in nature, from the formation of clouds to the behavior of marine life.