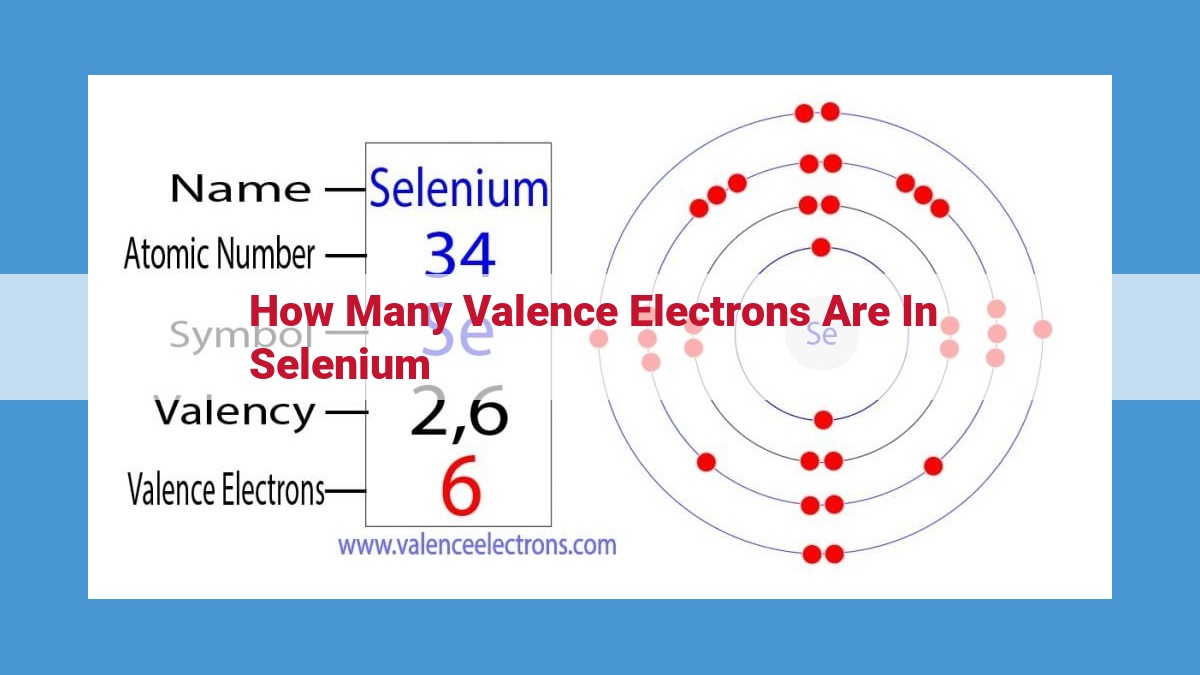
Selenium, an essential trace element in biological processes, exhibits six valence electrons. Valence electrons, located in the outermost energy level, are crucial for chemical reactivity. Selenium’s atomic number of 34 and electronic configuration (4s² 3d¹⁰ 4p⁴) reveal the presence of four valence electrons in its fourth energy level (4p⁴). These six valence electrons enable selenium to participate in chemical bonding, forming stable compounds with other elements. Understanding the number of valence electrons in selenium is fundamental to comprehending its reactivity and biological significance.
Selenium: The Vital Element for Life’s Tapestry
In the intricate dance of life, a remarkable element plays a pivotal role: selenium. This essential nutrient is a trace mineral found in all living organisms, from the tiniest bacteria to the mighty humans. Its presence is indispensable for a myriad of biological processes that are vital for our well-being.
Selenium is an antioxidant, safeguarding our cells from the ravages of harmful free radicals. It is a cofactor for glutathione peroxidase, a vital enzyme that detoxifies the body, protecting it from oxidative stress. Additionally, selenium is crucial for thyroid hormone metabolism, immune function, and DNA synthesis. Its versatility extends to supporting brain function, fertility, and even protecting against chronic diseases like cancer and cardiovascular ailments.
Understanding the Significance of Valence Electrons
In the realm of chemistry, understanding the concept of valence electrons is crucial for unraveling the mysteries of chemical bonding and reactivity. Valence electrons are the outermost electrons of an atom, merrily orbiting the atomic nucleus like celestial bodies in space. These electrons play a pivotal role in determining an element’s chemical properties and its ability to form bonds with other elements.
The Realm of Valence Electrons
Imagine an atom as a miniature universe, with the nucleus as its blazing sun and electrons as the planets orbiting around it. The outermost orbit, or valence shell, is where the valence electrons reside. These electrons are like the adventurous explorers of the atomic world, eager to venture out and interact with their neighbors.
The Importance of Valence Electrons in Bonding
Valence electrons are the key players in the dance of chemical bonding. When atoms meet, they seek to achieve stability by sharing or exchanging these outermost electrons. This bonding process creates molecules, the building blocks of all matter. The number and arrangement of valence electrons determine an element’s bonding capacity and the types of bonds it can form.
Reactivity: A Measure of Chemical Eagerness
The number of valence electrons also influences an element’s reactivity. Elements with a full valence shell, such as noble gases, are generally stable and unreactive. On the other hand, elements with incomplete valence shells, like metals, are more reactive and eager to share or gain electrons to achieve a stable configuration.
Understanding the concept of valence electrons is essential for comprehending the intricate world of chemical bonding and reactivity. By grasping the significance of these outermost electrons, we can unlock the secrets of how elements interact and form the substances that shape our physical world.
Understanding the Characteristics of Selenium: Delving into Its Atomic Structure
Selenium, an intriguing element with atomic number 34, holds a special place in the realm of chemistry. Its electronic configuration paints a vivid picture of its atomic structure, revealing intricate details that govern its unique properties.
At its heart lies the fourth energy level, the so-called valence shell, which houses selenium’s valence electrons. These six electrons, denoted as 4p⁴, play a pivotal role in shaping the element’s chemical behavior and reactivity.
Valence Electrons: Uncovering Selenium’s Chemical Secrets
Imagine selenium, an intriguing element with a story to tell. It’s a key player in biological processes, making it essential for life itself. But what’s the secret behind its remarkable abilities? The answer lies in its valence electrons.
Valence Electrons: The Gatekeepers of Chemical Reactions
Just like us humans have our unique personalities, atoms have their own electron configurations. Valence electrons are the electrons in an atom’s outermost energy level, and they determine how the atom interacts with others. In the case of selenium, it has six of these valence electrons, making it a reactive element.
Selenium’s Electronic Dance
Picture selenium’s outermost energy level, like a dance floor, where the valence electrons move freely. These four electrons are arranged in a specific way, 4p⁴, which means they occupy four out of the six possible orbitals. This electron arrangement gives selenium its distinctive chemical properties.
Selenium’s Chemical Behavior: A Reflection of Its Valence Electrons
Selenium’s six valence electrons make it eager to form bonds with other atoms. It can gain or lose electrons, or share them, which allows it to participate in various chemical reactions. This versatility explains why selenium is found in a wide range of compounds, from antioxidants to semiconductors.
Selenium’s six valence electrons are the driving force behind its chemical behavior. They determine its reactivity, its ability to form bonds, and its role in biological processes. Understanding valence electrons is the key to unlocking the secrets of this fascinating element.