To find unpaired electrons, start by determining the electron configuration using the periodic table and Aufbau Principle. Use Hund’s Rule to place electrons in degenerate orbitals, ensuring equal spins before pairing. Apply the Pauli Exclusion Principle to limit electron occupancy in each subshell to two. In molecular systems, utilize Molecular Orbital Theory to construct molecular orbitals and ascertain spin multiplicity using Hund’s Rule and the Pauli Exclusion Principle. By following these guidelines, you can accurately identify the number of unpaired electrons, which play key roles in magnetic properties, reactivity, and molecular bonding.
The Significance of Unpaired Electrons: A Tale of Magnetism, Reactivity, and Bonding
In the realm of chemistry, electrons dance in a delicate ballet, their movements and configurations shaping the very nature of matter. Unpaired electrons, those solitary dancers, play a pivotal role in this symphony, influencing a myriad of chemical phenomena.
These unruly electrons possess a unique property known as spin, which gives rise to their magnetic behavior. Like tiny magnets, they create a magnetic field, giving substances with unpaired electrons paramagnetic properties. This magnetism holds practical applications in fields such as magnetic resonance imaging (MRI), where the spin of unpaired electrons provides valuable insights into the structure and function of biological systems.
Unpaired electrons also wield a profound influence on a substance’s reactivity. They act as eager participants in chemical reactions, readily forming bonds with other atoms or molecules to achieve a more stable electron configuration. This enhanced reactivity makes compounds with unpaired electrons indispensable in various chemical processes, including catalysis, polymerization, and energy storage.
Moreover, the presence of unpaired electrons profoundly affects chemical bonding. In covalent bonding, where atoms share electrons to form molecules, unpaired electrons can form radical species, highly reactive intermediates that play crucial roles in many biochemical reactions and industrial processes.
Understanding the significance of unpaired electrons is essential for unraveling the complexities of chemical behavior. Join us as we delve into the fascinating world of these enigmatic dancers, revealing their profound impact on the world around us.
Hund’s Rule and the Aufbau Principle: Guiding Electron Distribution
In the realm of chemistry, electrons play a pivotal role, especially when they exist in an unpaired state. These electrons, with their inherent magnetic properties, reactivity, and bonding capabilities, hold immense significance in shaping the behavior of atoms and molecules.
To delve deeper into the fascinating world of unpaired electrons, we must turn to two fundamental principles that govern electron distribution: Hund’s Rule and the Aufbau Principle.
Hund’s Rule: Maximizing Spin Multiplicity
Hund’s Rule states that when electrons occupy degenerate orbitals (orbitals with the same energy), they distribute themselves in a way that maximizes the spin multiplicity of the system. Spin multiplicity refers to the number of possible spin configurations for a given number of electrons.
In other words, electrons prefer to have parallel spins, resulting in a higher spin multiplicity. This arrangement leads to a state of lower energy, enhancing the stability of the atom or molecule.
Aufbau Principle: Building from the Ground Up
The Aufbau Principle, on the other hand, dictates the order in which electrons fill atomic orbitals. It states that electrons occupy the lowest energy orbitals first, followed by higher energy orbitals. This principle ensures that atoms and molecules achieve their most stable electron configuration.
Unpaired Electrons: A Curious Consequence
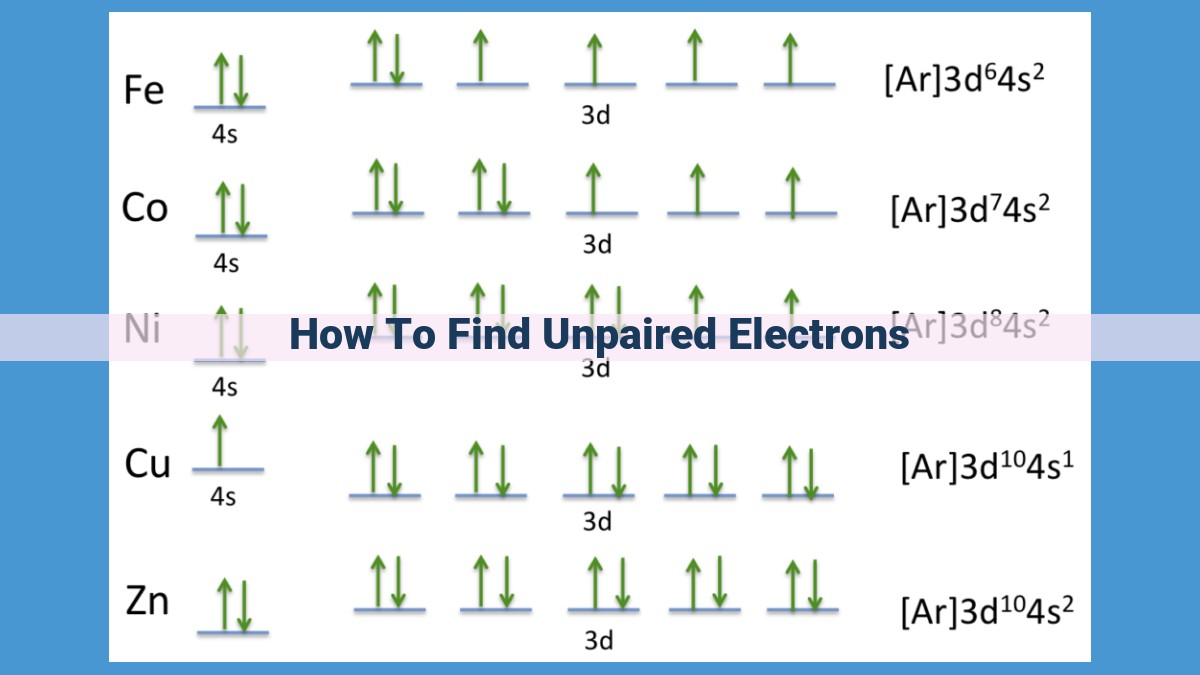
The interplay between Hund’s Rule and the Aufbau Principle often leads to the presence of unpaired electrons. When degenerate orbitals are only partially filled, electrons occupy them with parallel spins, resulting in unpaired electrons. This phenomenon is particularly common in transition metals, which frequently have unfilled d orbitals.
Understanding the distribution of electrons is crucial for predicting the properties and reactivity of chemical species. By embracing Hund’s Rule and the Aufbau Principle, we gain valuable insights into the behavior of these fundamental particles, unlocking a deeper comprehension of the chemical world.
Pauli Exclusion Principle: Ensuring Unique Electron Sets
- Describe the Pauli Exclusion Principle and its implications for electron occupancy in subshells.
- Explain how this principle limits the number of electrons per subshell and leads to unpaired electrons in degenerate orbitals.
The Pauli Exclusion Principle: Unraveling the Electron Dance
In the bustling city of an atom, electrons, the tiniest of dancers, follow a stringent set of rules dictated by the Pauli Exclusion Principle. This fundamental principle governs the arrangement of electrons within the atom’s energy levels, ensuring their unique identities and preventing them from tripping over each other.
The Pauli Exclusion Principle states that no two electrons within an atom can have the same set of quantum numbers. This means that electrons must differentiate themselves in three distinct ways: their energy level, their orbital shape, and their spin.
Each energy level, or shell, is divided into subshells, designated by the letters s, p, d, and f. Each subshell can accommodate a specific number of electrons, and it’s here that the Pauli Exclusion Principle comes into play. No more than two electrons can occupy the same subshell, and they must have opposite spins.
Spin refers to the intrinsic angular momentum of an electron, which can be either clockwise or counterclockwise. The Pauli Exclusion Principle dictates that these two electrons must have opposite spins. This ensures that electrons within the same subshell are as unique as snowflakes, each with its distinctive personality.
When degenerate orbitals, or orbitals with the same energy level, arise, the Pauli Exclusion Principle limits the number of electrons that can occupy them. Since each subshell can accommodate a maximum of two electrons, the presence of degenerate orbitals leads to the formation of unpaired electrons. These lone wolf electrons reside in their orbitals, eagerly awaiting their dance partners.
Understanding the Pauli Exclusion Principle is akin to unlocking a secret code that governs the intricate choreography of electrons within an atom. It’s a principle that ensures order, uniqueness, and the harmonious dance of the atomic world.
Molecular Orbital Theory and Spin Multiplicity: The Key to Unraveling Unpaired Electron Behavior in Molecules
As we delve into the fascinating world of molecular orbital theory, we uncover a powerful tool for comprehending how electrons behave within molecules. This theory provides an intricate framework that enables us to visualize the distribution of electrons and their energy levels, paving the way for a deeper understanding of chemical bonding.
Molecular Orbital Theory: A Quantum Leap in Electron Distribution
Molecular orbital theory paints a vivid picture of how electrons are dispersed within molecules. It introduces the concept of molecular orbitals, which are regions within a molecule where electrons are most likely to be found. These molecular orbitals originate from the overlap of atomic orbitals, the regions where electrons reside in atoms. The combination of atomic orbitals gives rise to molecular orbitals that exhibit distinct shapes and energy levels.
Spin Multiplicity: A Tale of Electron Spins
In the realm of quantum chemistry, electrons possess an intrinsic property known as spin. Electrons can either spin in the same direction (known as “parallel spin”) or in opposite directions (“antiparallel spin”). Spin multiplicity refers to the number of unpaired electrons within a molecule. Hund’s rule and the Pauli exclusion principle play crucial roles in determining the spin multiplicity.
Hund’s Rule: Maximizing Unpaired Electrons
Hund’s rule asserts that, for a given set of degenerate orbitals (orbitals with the same energy), electrons will occupy separate orbitals with parallel spins before pairing up in the same orbital with antiparallel spins. This rule drives the arrangement of electrons in a way that maximizes the number of unpaired electrons.
Pauli Exclusion Principle: Enforcing Electron Uniqueness
The Pauli exclusion principle declares that no two electrons within an atom or molecule can have the same set of four quantum numbers. This principle effectively limits the number of electrons that can occupy any given orbital to two, with their spins antiparallel. The interplay between Hund’s rule and the Pauli exclusion principle dictates the spin multiplicity of molecules, providing a foundation for understanding their magnetic properties and chemical reactivity.
Unveiling the Mystery of Unpaired Electrons
In the realm of chemistry, unpaired electrons hold profound significance, influencing a myriad of phenomena such as magnetism, reactivity, and bonding. Understanding their nature and distribution is crucial for unraveling the intricacies of chemical systems.
A Step-by-Step Guide to Identifying Unpaired Electrons
1. Electron Counting:
The first step in identifying unpaired electrons is tallying the total number of electrons in the system. This can be achieved by multiplying the atomic number of each element by the number of atoms present.
2. Electron Configuration:
With the total electron count established, the next step is to determine the electron configuration. This involves assigning electrons to their respective orbitals, starting with the lowest energy level. Orbitals are defined by their shape and energy and can hold a maximum of two electrons with opposite spins.
3. Hund’s Rule and Degenerate Orbitals:
Hund’s Rule states that electrons prefer to occupy degenerate orbitals, which are orbitals with the same energy, singly before pairing up. This means that electrons will spread out as much as possible to minimize their repulsive interactions.
4. Molecular Orbital Theory and Spin Multiplicity:
Molecular Orbital Theory describes electron distribution in molecules. When two atomic orbitals merge, they form molecular orbitals. In molecules, electrons are distributed among these molecular orbitals, with each orbital having a specific energy level and spin multiplicity. Spin multiplicity refers to the number of unpaired electrons in a molecule, which can influence its magnetic properties.
5. Unpaired Electron Count:
To ascertain the number of unpaired electrons, follow these steps:
a. Determine the electron configuration for the system.
b. Identify the degenerate orbitals with unpaired electrons (according to Hund’s Rule).
c. Count the number of orbitals with unpaired electrons to obtain the unpaired electron count.
Remember, unpaired electrons can have a profound impact on the behavior of atoms and molecules, making their identification a crucial step in understanding chemical systems. By following these steps, you can confidently unravel the mysteries surrounding unpaired electrons.