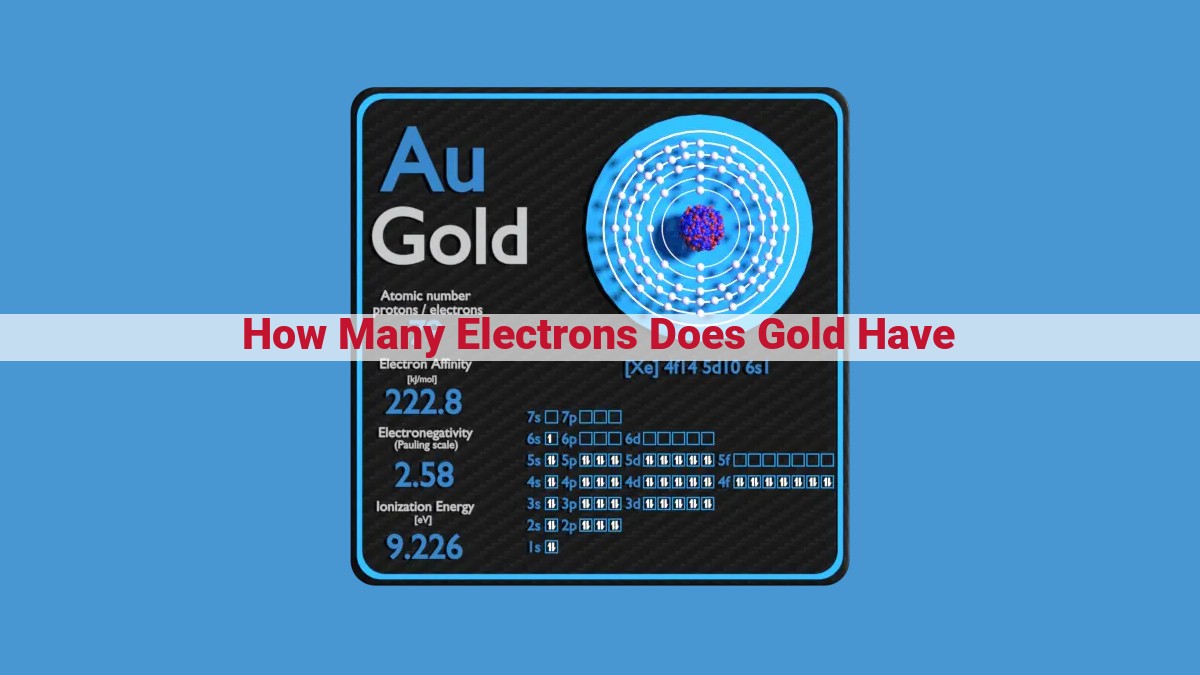
Gold, renowned for its value and luster, possesses 79 electrons, corresponding to its atomic number. This atomic structure comprises six electron shells, with the outermost shell containing one valence electron (6s¹). The electron configuration, 1s², 2s², 2p⁶, 3s², 3p⁶, 3d¹⁰, 4s², 4p⁶, 4d¹⁰, 5s², 5p⁶, 4f¹⁴, 5d⁹, 6s¹, reflects the orderly filling of atomic orbitals. Gold’s single valence electron contributes to its high electrical conductivity, making it a valuable material in electronics.
Significance of Gold and Atomic Structure
- Explain the importance of gold and introduce the concept of atomic structure and electron configuration.
Gold: A Journey through Its Atomic Structure
Throughout history, gold has captivated hearts and minds with its allure and rarity. This precious metal not only holds cultural and economic significance but also possesses fascinating characteristics at the atomic level. Gold’s unique atomic structure and electron configuration grant it remarkable properties that make it essential in various fields.
The significance of gold stems from its scarcity and its resistance to corrosion, making it a valuable commodity and an ideal material for jewelry and coinage. To fully understand gold’s properties, we must delve into the realm of atomic structure and electron configuration.
Atomic Structure: Unlocking the Secrets of Gold
Every element on Earth, including gold, is composed of tiny units called atoms. At the heart of each atom lies the nucleus, containing protons and neutrons. Surrounding the nucleus are electrons, which orbit in distinct energy levels or shells. The number of electrons in an atom defines its chemical identity and properties.
Gold’s Atomic Number: Unveiling Its Identity
Each element is assigned an atomic number, which represents the number of protons in its nucleus. Gold’s atomic number is 79, indicating that its nucleus contains 79 protons. This unique number classifies gold as an element distinct from all others.
Electron Configuration: Mapping Gold’s Electronic Landscape
The arrangement of electrons within an atom is known as its electron configuration. This configuration follows a set of rules known as the Aufbau principle, which dictates the order in which electrons fill atomic orbitals. Gold’s complete electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 4f¹⁴ 5d⁹ 6s¹
Valence Electrons: The Key to Gold’s Reactivity
Valence electrons are the electrons occupying the outermost shell of an atom. These electrons determine an element’s chemical reactivity and bonding capabilities. Gold has a single valence electron in its 6s orbital, giving it a relatively inert nature. This low reactivity contributes to gold’s corrosion resistance and makes it a valuable material for jewelry and electronics.
Gold’s Electronic Properties: Shining Bright in the World of Technology
Gold’s electronic configuration also explains its remarkable conductivity, making it an excellent material for electrical applications. The loosely held valence electron allows gold to conduct electricity efficiently. Moreover, gold’s resistance to oxidation preserves its conductivity over extended periods. As a result, gold is widely used in electronics, from circuits to connectors, ensuring reliable and efficient performance.
Gold’s significance extends beyond its monetary value to its fascinating atomic structure and electron configuration. Understanding the number of protons, electrons, and their arrangement unveils the secrets behind gold’s unique properties. These properties, including its corrosion resistance, high conductivity, and inertness, make gold an essential element in various fields, from jewelry and coinage to electronics and technology.
Gold’s Atomic Number
- Define atomic number and explain its significance.
- State gold’s atomic number (79) and its implications for its classification as an element.
Gold’s Identity Revealed: Unraveling Its Atomic Number
In the glittering world of precious metals, gold stands out as a true treasure. But beneath its gleaming surface lies a secret that determines its very essence: its atomic number.
Let’s begin with a tale of atoms, the building blocks of all matter. Each atom possesses a unique identity, a fingerprint of sorts, defined by its atomic number. This number represents the amount of protons, positively charged particles, found within the atom’s nucleus.
Gold, the precious metal we hold dear, boasts an atomic number of 79. This number, etched into the very fabric of its existence, classifies gold as an element all its own. It marks its place on the periodic table, separating it from the vast array of other elements that grace our universe.
The atomic number of gold is not merely a random attribute; it shapes its being in profound ways. It determines the number of protons in its nucleus, which in turn dictates the number of electrons that orbit around it. And the arrangement of these electrons, known as electron configuration, plays a pivotal role in defining gold’s properties.
So, when we admire the lustrous glow of gold, we are gazing not only at its physical beauty but also at the secrets encoded within its atomic structure. Its atomic number, a seemingly simple number, underpins the very essence of this precious metal, making it the coveted treasure it is.
Unveiling Gold’s Electron Configuration: A Journey into Its Atomic Structure
Delve into the captivating world of atomic structure and unravel the significance of gold, a precious metal revered throughout history. Its unique properties and applications stem from its intricate electron configuration, which holds the key to understanding its behavior and chemistry.
The Concept of Electron Configuration
An element’s electron configuration describes the arrangement of its electrons within atomic orbitals, which are regions of space surrounding the nucleus where electrons reside. This configuration profoundly influences an element’s chemical and physical properties.
Gold’s Atomic Number
Gold possesses an atomic number of 79, indicating that its nucleus contains 79 protons. This number is an elemental fingerprint, distinguishing it from all other elements and determining its position on the periodic table.
Aufbau Principle and Filling Atomic Orbitals
Electrons fill atomic orbitals in a specific order, following the Aufbau principle. Each orbital can hold a maximum of two electrons. Starting from the lowest energy level, electrons progressively occupy orbitals until the atom reaches its unique electron configuration.
Gold’s Complete Electron Configuration
Gold’s electron configuration is (1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 4f¹⁴ 5d⁹ 6s¹). This seemingly complex notation represents the distribution of its 79 electrons, which are organized into six concentric energy levels, or shells.
Number of Valence Electrons
Valence electrons are those occupying the outermost energy level, and they play a crucial role in chemical reactions and bonding. Gold has one valence electron in its 6s orbital. This lone electron determines its reactivity and bonding capabilities.
Electron Shell Structure
Gold’s electron configuration further reveals its electron shell structure, which is 2, 8, 18, 32, 18, 1. This distribution shows that gold has six electron shells, with the outermost shell containing only one electron. The arrangement of electrons in these shells influences gold’s physical and chemical properties, such as its high density and malleability.
Understanding gold’s electron configuration is fundamental to comprehending its exceptional qualities. The number and arrangement of its electrons dictate its chemical reactivity, conductivity, and other distinctive properties. This knowledge paves the way for harnessing gold’s unique traits in diverse applications, from jewelry and electronics to advanced scientific research.
Unraveling the Enigmatic Electron Configuration of Gold: Unveiling the Significance of Valence Electrons
Gold, an element shrouded in allure and mystery, has played a pivotal role in shaping civilizations for millennia. Its distinctive properties, including its alluring luster and exceptional conductivity, stem from the intricate dance of electrons within its atomic structure. In this exploration, we delve into the enigmatic world of gold’s electron configuration, focusing specifically on the significance of its valence electrons.
Valence Electrons: The Gatekeepers of Chemical Bonding
“Valence electrons,” the outermost electrons in an atom’s shell, are pivotal in determining an element’s chemical reactivity. They dictate the atom’s ability to form bonds with other atoms, shaping its chemical nature and ability to participate in countless reactions. In the case of gold, a single valence electron resides in its outermost shell, denoted as 6s¹. This lone electron holds the key to understanding gold’s unique chemical behavior.
Gold’s Lone Electron: A Catalyst for Noble Status
Gold’s valence electron configuration of 6s¹ has profound implications for its chemical reactivity. This single electron imparts a noble character to gold, rendering it less prone to forming chemical bonds. Gold’s reluctance to react with other elements contributes to its renowned stability and resistance to corrosion, traits that have made it a coveted material throughout history.
The Dance of Electrons: Gold’s Conductivity Unveiled
Beyond its chemical properties, gold’s valence electrons also play a crucial role in its remarkable electrical conductivity. The ability of electrons to move freely within the gold atom’s structure allows gold to conduct electricity with great efficiency. This exceptional property has made gold a vital component in countless electronic devices, from intricate circuit boards to high-performance connectors.
In conclusion, the valence electrons of gold, though seemingly inconspicuous, profoundly shape the element’s behavior. Their influence extends from its chemical reactivity to its exceptional conductivity, underscoring the profound impact of electron configuration on the properties of matter. Understanding the intricacies of gold’s electron configuration provides a deeper appreciation for this precious metal’s unique characteristics and its indispensable role in our technological landscape.
Electron Shell Structure of Gold
In the captivating realm of chemistry, the intricacies of an element’s atomic structure shape its destiny, determining not only its appearance but also its myriad properties. Among the elements, gold stands out as a gleaming symbol of wealth and beauty, its intrinsic value stemming from its unique electron configuration.
As we delve into gold’s atomic blueprint, we encounter the concept of electron shells, concentric layers of orbitals encircling the atomic nucleus. Each shell can hold a finite number of electrons, and their arrangement within these shells profoundly influences an element’s behavior.
Gold’s electron shell structure is a tale of six shells, a harmonious arrangement of 2, 8, 18, 32, 18, and 1 electrons. This unique distribution has far-reaching implications, shaping gold’s physical and chemical characteristics.
The outermost shell, with its solitary electron, plays a crucial role in determining gold’s reactivity. This lone electron is eager to form bonds, endowing gold with the capacity to interact with other elements. However, gold’s filled inner shells shield the nucleus effectively, making it resistant to oxidation, a property that has made gold an enduring symbol of permanence.
The harmonious balance of gold’s electron shell structure also contributes to its high electrical conductivity. Electrons flow effortlessly through gold’s atomic structure, making it an ideal choice for electrical applications. This property underpins gold’s widespread use in electronics, where it finds its place in everything from delicate circuit boards to gleaming connectors.
By unraveling the secrets of gold’s electron shell structure, we gain a profound understanding of this precious metal’s captivating properties. From its lustrous shine to its exceptional conductivity, gold’s atomic architecture lies at the heart of its enduring allure.
Understanding the Electronic Marvel of Gold
Gold, a precious metal with an allure that has captivated civilizations for millennia, possesses remarkable electronic properties that contribute to its unique nature.
Exceptional Conductivity: The Flow of Electrons
Gold ranks among the most conductive metals, allowing electrons to flow through it with remarkable ease. This exceptional conductivity arises from the metal’s atomic structure. Gold’s atoms have a single electron in their outermost shell, known as a valence electron. This valence electron is loosely bound to the atom’s nucleus, allowing it to move freely throughout the metal lattice.
Noble Metal Status: Resistance to Oxidation
Gold’s single valence electron grants it another significant property: nobility. Noble metals are those that resist tarnishing or oxidation when exposed to air and moisture. In gold’s case, its single valence electron is tightly bound to the atom’s positively charged nucleus, making it difficult for other atoms to interact with it. This resistance to oxidation preserves gold’s lustrous appearance and its value as a precious metal.
Electronics Applications: Durability and Precision
The combination of gold’s high conductivity and nobility makes it an ideal material for various electronic applications. Gold’s resistance to oxidation ensures that electrical connections remain reliable and corrosion-free over time. This durability is crucial in high-precision electronic devices, such as printed circuit boards and connectors.
Gold’s high conductivity also makes it valuable for applications where precise current flow is critical. For instance, gold is used in high-performance wires and electrical contacts. Its ability to conduct electricity efficiently minimizes power loss and maintains signal integrity, essential for demanding applications such as telecommunications and data processing.
In conclusion, gold’s electronic properties are a testament to its unique atomic structure. Its high conductivity, nobility, and durability make it a valuable metal in various electronic applications, ranging from precision devices to high-performance circuits. Understanding these properties helps us appreciate the significance of gold beyond its traditional uses as a symbol of wealth and ornamentation, revealing its hidden role in the modern technological landscape.