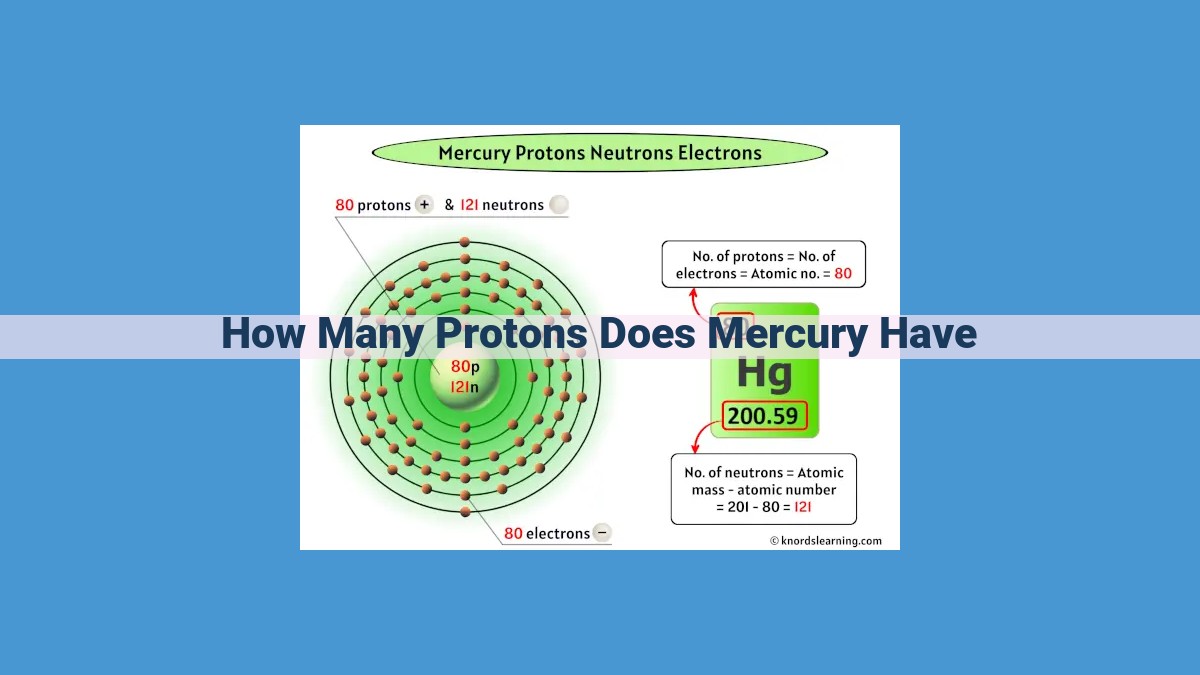
Mercury atoms possess a distinctive number of protons, which plays a crucial role in understanding their properties. The atomic number, a unique identifier for each element, determines the proton count. Mercury’s atomic number is 80, indicating that each mercury atom has 80 protons. These protons reside in the nucleus, contributing to the element’s positive charge and influencing its chemical behavior.
Unveiling the Secrets of Mercury: Exploring the Proton Count
In the realm of chemistry, understanding the fundamental structure of atoms is paramount. Among these building blocks of matter, the proton count holds significant importance, shaping an element’s identity and dictating its properties. In this blog post, we delve into the intriguing world of mercury atoms, exploring their proton count and its profound implications.
The Significance of Proton Count
The proton count, often referred to as the atomic number, defines the unique identity of each element. It represents the number of positively charged particles, or protons, found within the nucleus of an atom. This count not only determines an element’s position on the periodic table but also governs its chemical reactivity, physical properties, and overall behavior.
Atomic Number: The Gateway to Understanding Proton Count in Mercury
In the realm of chemistry, understanding the inner workings of elements is crucial for unraveling their properties and behavior. Among these elements, mercury stands out with its unique characteristics, and one key aspect that defines its identity is the number of protons within its atoms.
Embarking on the Atomic Number Journey
Every element in the periodic table is assigned an atomic number, a fundamental value that holds the key to unlocking the proton count. It represents the number of positively charged protons residing in the nucleus, the central core of an atom. Each element has a unique atomic number, acting as its chemical fingerprint.
Decoding Mercury’s Atomic Signature
In the case of mercury, its atomic number is 80. This means that at the heart of every mercury atom lies 80 protons, each carrying a single positive charge. This atomic number not only defines mercury’s identity but also serves as a guide to its other atomic properties.
The Significance of Atomic Number
The atomic number plays a pivotal role in determining not just the proton count but also the electronic configuration of an element. It dictates the number of electrons, which are negatively charged particles orbiting the nucleus. This electronic configuration, in turn, shapes the element’s chemical reactivity and overall behavior.
Implications for Mercury’s Chemistry
Understanding mercury’s proton count has profound implications for unraveling its chemical properties. The 80 protons in its nucleus give it a positive nuclear charge of +80, which attracts negatively charged electrons to form a neutral atom. This nuclear charge influences how mercury interacts with other elements, determining its chemical reactivity and bonding characteristics.
Unveiling the Proton Count Mystery
In essence, mercury’s atomic number of 80 reveals that each mercury atom contains 80 protons. This fundamental knowledge serves as a cornerstone for understanding mercury’s chemistry and its unique role in the world of elements.
Proton Count in Mercury: Unveiling the Number of Protons in a Liquid Metal
Imagine a silvery liquid, shimmering in its unique form—this is mercury, an element that fascinates with its unusual properties. To truly understand its essence, we must delve into the heart of its atoms, where the tiny protons reside. The number of protons in an atom defines its identity and governs its behavior.
Atomic Number: The Gateway to Proton Count
Every atom is characterized by its atomic number, a number unique to each element. It represents the number of protons found in the nucleus—the atom’s central hub. Mercury, with an atomic number of 80, boasts 80 protons within its nucleus.
Proton Population in Mercury’s Nucleus
The protons, along with the neutrons, reside in the nucleus. Mercury’s atomic number of 80 directly translates to 80 protons. This proton population plays a crucial role in shaping the element’s properties.
Proton Configuration: The Dance of Protons
Protons are not randomly dispersed within the nucleus; they follow a specific arrangement known as the proton configuration. This intricate dance of protons influences an element’s chemical and physical behavior. Understanding mercury’s unique proton configuration allows us to unravel its remarkable characteristics.
Nuclear Charge: The Power of Protons
The cluster of protons in the nucleus generates a positive charge, known as the nuclear charge. Mercury’s nuclear charge of +80 reflects the collective charge of its 80 protons. This nuclear charge influences interactions with other atoms, determining the element’s reactivity and bonding nature.
Mercury’s identity stems from its 80 protons, a number revealed by its atomic number. Delving into the proton count and configuration unveils the element’s remarkable properties, paving the way for a deeper understanding of this fascinating liquid metal.
Proton Configuration: The Arrangement of Mercury’s Nuclear Residents
Mercury, with its silvery sheen and enigmatic nature, holds a trove of secrets within its atomic structure. At its core, the arrangement of protons plays a crucial role in shaping its unique characteristics.
The Dance of Protons: Understanding Proton Configuration
Within the heart of every mercury atom, a dynamic dance of protons unfolds. These positively charged particles, the building blocks of the atomic nucleus, are arranged in a specific configuration that defines the element’s identity. For mercury, this configuration is aptly described by the sequence 1s2 2s2 2p6…
Each number in this sequence represents an energy level or shell, while the letter (s, p) denotes the type of orbital within that shell. The superscripts indicate the number of protons occupying each orbital.
Impact on Mercury’s Attributes: The Invisible Puppeteer
The proton configuration of mercury has a profound impact on its chemical and physical properties. It governs the element’s reactivity, bonding behavior, and even its magnetic properties.
For instance, the presence of two valence electrons in the outermost shell makes mercury a poor conductor of electricity. Its high density and low melting point, on the other hand, are attributed to the strong electrostatic interactions between the protons and electrons.
Unlocking the Secrets of Atomic Architecture
Understanding the proton configuration of mercury is not merely an academic pursuit. It’s the key to unraveling the mysteries of this fascinating element. From its use in thermometers and barometers to its potential applications in superconductivity, a deep dive into mercury’s atomic structure opens up a world of possibilities.
So, next time you encounter mercury in your daily life or scientific endeavors, remember the intricate dance of protons within its nucleus. It’s this hidden microcosm that shapes the element’s unique properties and makes it an indispensable part of our technological landscape.
Nuclear Charge: The Positive Force of Protons
The nucleus, the heart of an atom, houses a multitude of protons and neutrons. Protons, carrying a positive electrical charge, play a crucial role in defining the identity and behavior of an element. In the case of mercury, a fascinating element with unique properties, the number of protons in its nucleus is a key factor in unraveling its secrets.
Mercury’s atomic number, a fundamental property that distinguishes it from all other elements, is 80. This number represents the total number of protons residing within the nucleus. The atomic number serves as a direct indicator of the proton count, as each element possesses a unique atomic number that corresponds to its specific number of protons.
The nuclear charge, symbolized by Z, is the net positive charge of the atomic nucleus. For mercury, with 80 protons, the nuclear charge is +80. This positive charge exerts a strong electrostatic force, attracting negatively charged electrons to form a neutral atom.
The nuclear charge plays a significant role in various atomic interactions. It determines the chemical properties of an element by influencing the interactions between electrons and the nucleus. It also affects the ionization energy, the energy required to remove an electron from the atom. Elements with a higher nuclear charge, like mercury, generally have higher ionization energies, making it more difficult to remove electrons and form ions.
Moreover, the nuclear charge influences the stability of an atom. Atoms with a balanced number of protons and electrons are stable, while those with an imbalance may be unstable, leading to radioactive decay. Mercury’s relatively high nuclear charge contributes to its stability, making it a relatively long-lived element.
Understanding the nuclear charge of an element, like mercury, provides valuable insights into its atomic structure, chemical behavior, and overall properties. The proton count, represented by the atomic number, and the resulting nuclear charge are fundamental characteristics that shape the unique identity of each element in the periodic table.