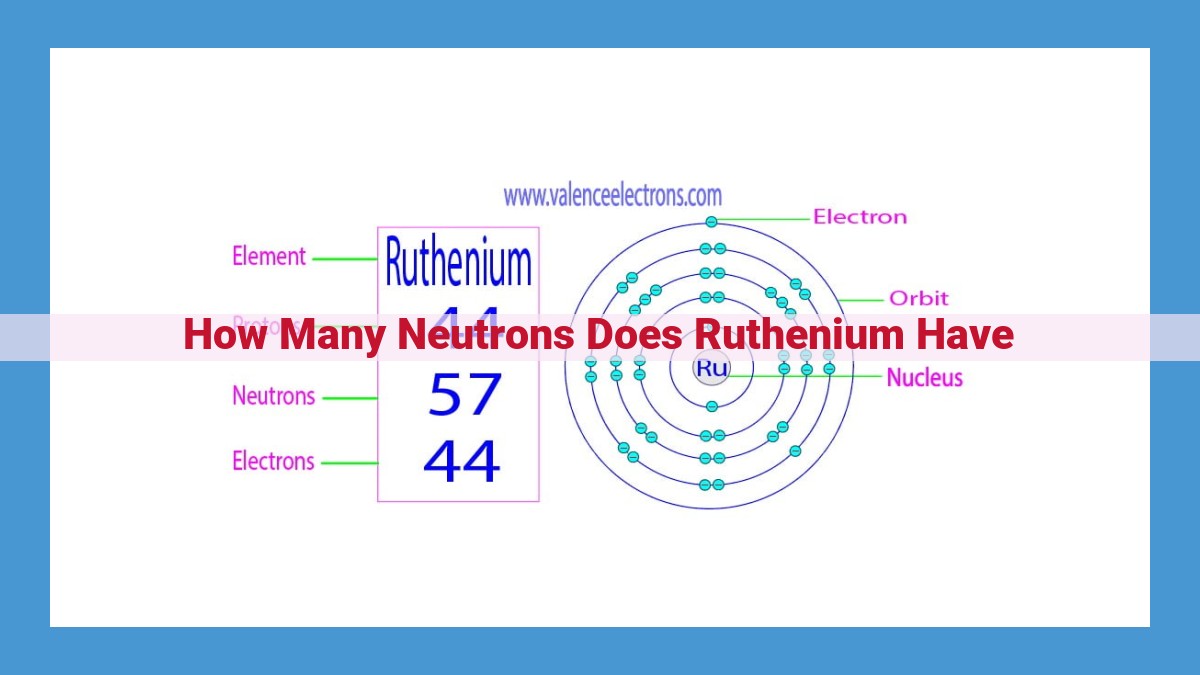
Ruthenium, with an atomic number of 44, possesses a mass number of 101.26, indicating its average mass. To determine the number of neutrons, we subtract the atomic number from the mass number, resulting in approximately 57.26 neutrons in the nucleus of ruthenium. However, due to the existence of isotopes, the exact number of neutrons can vary, affecting the isotope’s mass and stability.
How Many Neutrons Does Ruthenium Have? A Journey to the Heart of the Atom
In the vast expanse of the periodic table, there lies a metal of exceptional allure and significance: Ruthenium. This enigmatic element, emblazoned with the atomic symbol Ru and atomic number 44, has captivated the minds of scientists and captivated the imagination of enthusiasts alike. One of its most intriguing characteristics is its elusive neutron count, a question that has sparked curiosity and ignited scientific exploration for decades. Embark on a captivating quest to unravel this mystery, uncovering the secrets that lie at the core of ruthenium’s atomic structure.
Ruthenium, a precious metal renowned for its strength, corrosion resistance, and shimmering silver-white appearance, has found widespread applications in diverse industries, from jewelry and electronics to dentistry and aerospace. Its versatility stems from its unique properties, which are deeply intertwined with the fundamental makeup of its atoms.
At the heart of every ruthenium atom lies a nucleus, a compact and densely packed region that harbors the element’s protons and neutrons. Protons, positively charged particles, define an element’s identity and determine its atomic number. Ruthenium, with an atomic number of 44, boasts 44 protons within its nucleus, establishing its position on the periodic table.
Alongside protons, neutrons also reside within the nucleus, contributing to the atom’s mass but carrying no electrical charge. The number of neutrons in an atom, coupled with the number of protons, determines its mass number. Ruthenium’s mass number, 101.26, signifies the average mass of its atoms, taking into account the varying numbers of neutrons among its isotopes.
To unveil the elusive number of neutrons in ruthenium, we employ a simple yet elegant calculation: subtracting the atomic number from the mass number. This mathematical maneuver reveals that ruthenium’s nucleus harbors approximately 57.26 neutrons, contributing to its overall mass and shaping its physical and chemical properties.
Ruthenium’s neutron count is not a fixed value but rather a spectrum of possibilities, as the element exists as a mixture of isotopes. Isotopes are atoms of the same element that share the same atomic number but differ in their neutron count. This variation in neutron number affects the isotope’s mass, influencing its stability and prevalence in nature.
As we delve deeper into the world of ruthenium isotopes, we encounter a fascinating array of these atomic variations, each with its unique characteristics and applications. Their diverse neutron counts give rise to a range of masses, influencing the element’s overall properties and expanding its versatility.
In conclusion, the number of neutrons in ruthenium is an intriguing aspect of this remarkable element’s atomic structure. With approximately 57.26 neutrons residing in its nucleus, ruthenium’s mass number of 101.26 reflects its unique combination of protons and neutrons. The presence of multiple isotopes further enriches the element’s properties, granting it a spectrum of applications that span a multitude of industries. As we continue to explore the fascinating world of atoms and elements, the secrets of ruthenium and its neutron count continue to captivate our minds and inspire scientific advancements.
How Many Neutrons Does Ruthenium Have? Unraveling the Secrets of This Element
Ruthenium, an element often thought of as mysterious and obscure, holds a captivating story within its atomic structure. This intriguing element, symbolized by the letters Ru and bearing the atomic number 44, invites us on an enlightening journey into the realm of neutrons.
Atomic Number: The Key to Unlocking the Protons
Every element possesses a unique atomic number, a fundamental number that defines its identity. This number, like a secret code, reveals the number of protons residing within the atom’s nucleus, the very heart of its existence. For ruthenium, this atomic number stands at 44, indicating the presence of 44 protons, the building blocks of this element’s nucleus.
Unveiling the Mass Number: A Journey into the Core
Beyond the atomic number lies another crucial number, the mass number, which represents the total number of protons and neutrons found within the atom’s nucleus. Ruthenium, with its mass number of 101.26, invites us to delve deeper into its inner workings. This number, an average representation of its atomic mass, hints at the presence of additional particles beyond the 44 protons.
Determining Neutron Count: Subtracting Protons from Mass
Neutrons, elusive yet essential particles, share the nucleus with protons, contributing to an atom’s mass. To unravel the mystery of ruthenium’s neutron count, we embark on a simple calculation, subtracting the atomic number (44) from the mass number (101.26). This mathematical dance reveals that ruthenium harbors approximately 57.26 neutrons, companions to the 44 protons, forming the very essence of this element.
Isotopes of Ruthenium: A Tapestry of Variety
Ruthenium’s story takes an unexpected turn when we explore its isotopic diversity. Isotopes, variants of the same element, share the identical atomic number but differ in their neutron count, resulting in unique mass numbers. Ruthenium exists as a constellation of isotopes, each with its own distinctive neutron configuration, affecting their mass and stability, adding another layer to this element’s enigmatic nature.
How Many Neutrons Does Ruthenium Have?
Unveiling the Secrets of the Noble Metal
In the realm of chemistry, ruthenium stands as a captivating element with a rich history and intriguing properties. It’s essential to delve into its atomic structure to grasp its true nature, and a crucial aspect of that is understanding the number of neutrons it possesses. Embark on a storytelling journey as we embark on this scientific exploration.
The Significance of the Atomic Number
Every element on the periodic table is distinguished by its unique atomic number, which represents the number of protons residing in its atomic nucleus. These protons carry a positive charge and contribute to the element’s identity. In the case of ruthenium, it proudly boasts an atomic number of 44, signifying the presence of 44 protons.
Unraveling the Mass Number
The mass number, a fundamental characteristic of an element, encapsulates the total number of protons and neutrons nestled within the nucleus. For ruthenium, scientists have meticulously determined its mass number to be approximately 101.26. This intriguing value hints at the presence of a substantial number of neutrons.
Uncovering the Number of Neutrons
Intriguingly, the number of neutrons in an element’s nucleus is not explicitly stated in its elemental makeup. Instead, it can be skillfully calculated by subtracting the atomic number from the mass number. With ruthenium’s atomic number of 44 and mass number of 101.26, simple arithmetic reveals the presence of approximately 57.26 neutrons.
Embracing the Diversity of Isotopes
Ruthenium, like many elements, manifests itself in a myriad of isotopic forms, each with a distinct neutron count. These isotopes share the same atomic number, but their varying neutron numbers endow them with different masses and stability characteristics. This isotopic diversity enriches the element’s chemistry and expands its applications in various fields.
How Many Neutrons Does Ruthenium Have? A Comprehensive Exploration
Unveiling Ruthenium’s Atomic Structure
In the realm of chemical elements, ruthenium stands out with its unique atomic characteristics. Let’s embark on a journey to discover the number of neutrons that reside within its nucleus.
The Concept of Atomic Number
Every element is defined by its atomic number, which represents the number of protons in its nucleus. Protons carry a positive electrical charge. Ruthenium proudly boasts an atomic number of 44, indicating the presence of 44 protons in its core.
Mass Number: Uniting Protons and Neutrons
The mass number of an element encompasses the total number of protons and neutrons in its nucleus. Ruthenium has a mass number of 101.26, indicating an average mass of its atoms.
Determining the Number of Neutrons
To determine the number of neutrons in ruthenium, we invoke a simple calculation: neutrons = mass number – atomic number. Subtracting ruthenium’s atomic number (44) from its mass number (101.26), we arrive at approximately 57.26 neutrons.
This result suggests that ruthenium’s nucleus contains approximately 57 neutrons, contributing significantly to its overall mass.
Isotopes of Ruthenium
Ruthenium’s Isotopic Symphony
Ruthenium, a fascinating chemical element, isn’t just a static entity. It comes in different forms, known as isotopes, each with a unique tale to tell. Isotopes are like siblings within the ruthenium family, sharing the same atomic number (44) but differing in their neutron count.
The Neutron Dance
Neutrons, along with protons, reside at the heart of an atom, the nucleus. While protons determine an element’s identity, neutrons play a crucial role in shaping its mass and stability. Ruthenium’s mass number, 101.26, indicates its average mass. But dig a little deeper, and you’ll find that this number is not a fixed value.
Calculating Neutron Abundance
To unveil the mystery of how many neutrons grace ruthenium’s nucleus, we embark on a simple yet elegant calculation. We subtract the atomic number (44) from the mass number (101.26), and behold, we discover that on average, ruthenium boasts approximately 57.26 neutrons.
The Isotopic Spectrum
Ruthenium doesn’t exist as a solitary isotope; it’s a symphony of many. Like a musical ensemble, each isotope contributes its own unique note to the overall composition. These isotopic variations arise from the differing number of neutrons they harbor.
Stability and Mass
The number of neutrons profoundly influences an isotope’s stability and mass. Isotopes with a balanced neutron-to-proton ratio tend to be more stable, while those with an imbalance may face a turbulent existence. Moreover, as the neutron count increases, so does the isotope’s mass, creating a diverse range of isotopic masses within the ruthenium family.