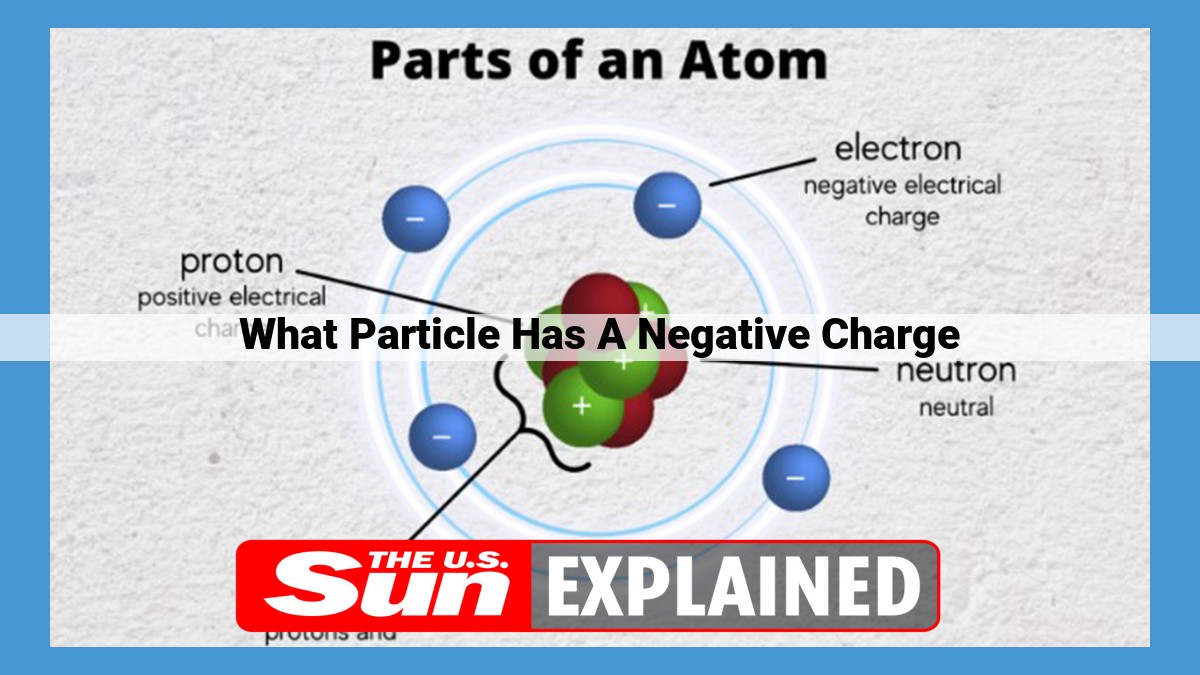
Electrons, fundamental subatomic particles, possess a negative electrical charge. They are extremely lightweight with a spin of 1/2. Electrons occupy energy levels within atoms, influenced by quantum mechanics. Their negative charge plays a crucial role in chemical reactions and in the behavior of materials.
Introduction:
- Briefly introduce the topic by stating that electrons have a negative charge.
Electrons: The Negatively Charged Building Blocks of Matter
In the vast tapestry of the universe, tiny particles known as electrons play a fundamental role in shaping the world around us. These subatomic constituents possess a unique attribute that sets them apart: a negative electrical charge.
Electrons are the building blocks of atoms, the basic units of matter. Each atom consists of a nucleus, which contains positively charged protons and neutral neutrons, surrounded by a swarm of negatively charged electrons. The balance between these positive and negative charges gives atoms their electrical neutrality.
The negative charge of electrons has a profound impact on the behavior of matter. It allows electrons to interact with other charged particles, both positively and negatively. This interaction forms the basis of chemical bonds, the forces that hold atoms together to create molecules and compounds.
The presence of negatively charged electrons also gives rise to electricity. When electrons flow through a conductor, such as a wire, they create an electric current. This current powers everything from our lights to our computers.
Electrons play a crucial role in many scientific fields. In chemistry, they determine the chemical properties of elements and their ability to form compounds. In physics, they are essential for understanding the behavior of electricity, magnetism, and the fundamental forces of nature. And in materials science, they are manipulated to create new materials with tailored properties.
As we delve deeper into the world of electrons, we will explore their mass, spin, and quantum mechanical behavior. We will also discuss how these properties influence the formation of atoms, molecules, and the universe itself.
Electrons: The Building Blocks of Our Universe
In the vast tapestry of the cosmos, the electron reigns as a fundamental particle, holding sway over the intricate workings of our universe. With its inherent negative charge, this elusive entity shapes the very fabric of reality.
Electron: A Subatomic Enigma
At the heart of every atom, electrons dance in a captivating symphony of motion. These subatomic particles are the cornerstone of matter, possessing an unyielding negative electrical charge. This charge, the foundation of all electricity, gives electrons their unique identity and distinguishes them from other subatomic inhabitants.
The Curious Case of Mass and Spin
While electrons are incredibly lightweight compared to their atomic counterparts, they possess a mysterious property known as spin. This intrinsic angular momentum, like the whirl of a celestial body, imparts a unique characteristic to electrons. Despite being infinitesimally small, electrons exhibit a duality of both particle and wave-like behavior, a testament to the enigmatic nature of quantum mechanics.
Delving into the Quantum Realm: Unveiling the Secrets of Electrons
Beyond their fundamental nature as negatively charged subatomic particles, electrons also inhabit the mysterious realm of quantum mechanics, where their behavior defies everyday experiences.
In this realm, electrons dance within energy levels, which are specific energy states they can occupy within an atom. These levels are not continuous but exist as discrete steps, much like the rungs of a ladder. Each level can accommodate a certain number of electrons, arranged in orbitals.
Atomic orbitals are three-dimensional regions around the nucleus where electrons are most likely to be found. They come in different shapes and orientations, such as s, p, and d orbitals. The distribution of electrons within orbitals is fundamental to understanding the chemical properties of elements.
For instance, an electron in a lower energy level is more tightly bound to the nucleus than one in a higher energy level. This difference in energy affects the reactivity and bonding behavior of atoms, shaping the world around us at the molecular level.
Unveiling the Enigma of Electrons: Delving into the Quantum Realm
Electrons, the subatomic particles that carry a negative charge, play a pivotal role in shaping our physical world. Their intricate properties and behaviors have fascinated scientists for centuries, leading to groundbreaking discoveries in the realms of chemistry, physics, and materials science.
Charge and Mass: Unveiling the Negative Charge Carriers
Electrons possess an intrinsic negative electrical charge, which is the foundation of their interactions with other charged particles. This negative charge is a defining characteristic that sets electrons apart from other subatomic particles, such as protons and neutrons. Interestingly, despite their negative charge, electrons are remarkably lightweight. In fact, their mass is about 1/1836th of a proton, making them the lightest charged particles known to science.
Spin: A Unifying Dance of Angular Momentum
Electrons also possess an intrinsic angular momentum, known as spin. This spin can be visualized as a tiny whirling motion that imparts a magnetic field to the electron. It is a fundamental property that distinguishes electrons from other particles and plays a crucial role in their behavior within atoms and molecules.
Quantum Mechanics: Unlocking the Secrets of Electron Behavior
To fully grasp the enigmatic nature of electrons, we must embrace the principles of quantum mechanics. This theory governs the behavior of particles at the atomic and subatomic levels and provides a framework for understanding the peculiar behaviors of electrons.
Energy levels are specific energy states that electrons can occupy within an atom. Electrons occupy the lowest energy states, known as orbitals, which are three-dimensional regions where electrons are most likely to be found. These orbitals have distinct shapes and energies, influencing the electron’s distribution and interactions within the atom.