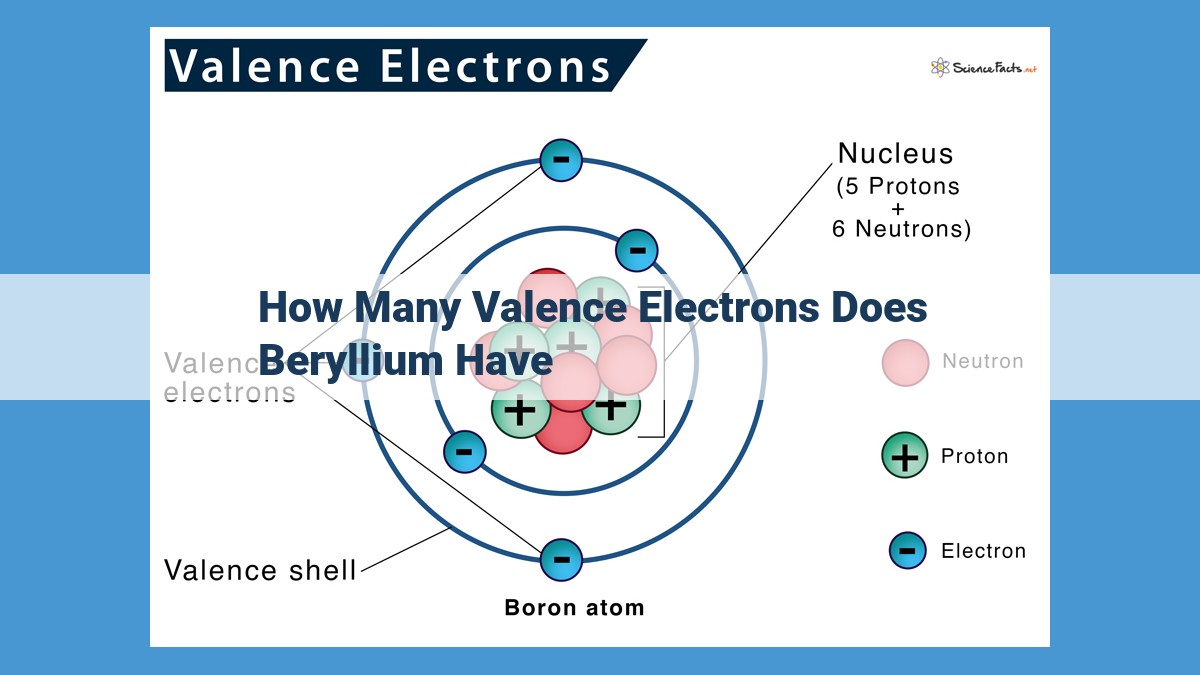
Beryllium possesses four electrons, with two located as valence electrons in its outermost electron shell. These valence electrons occupy the 2s orbital and play a crucial role in determining the chemical properties and reactivity of the element. Beryllium’s low number of valence electrons makes it prone to forming ionic bonds, highlighting the significance of valence electrons in defining chemical behavior.
Atomic Structure of Beryllium
- Explain the atomic number (4) and atomic mass (9.012182) of beryllium.
Delving into the Atomic Realm of Beryllium: Unveiling Its Structure and Reactivity
Embark on a captivating journey into the atomic realm of beryllium, an element that holds secrets to its unique chemical properties. Atomic number and atomic mass are the fundamental building blocks of atomic identity, and beryllium proudly bears the atomic number 4, denoting the presence of 4 protons in its nucleus. Its atomic mass of 9.012182 reflects the sum of its protons and neutrons, which collectively define its mass.
Unraveling the Significance of Valence Electrons
Electrons, the fundamental particles that dance around the atomic nucleus, are not all created equal. Valence electrons, like the extroverted members of a social circle, reside in the outermost electron shell. They play a pivotal role in shaping an atom’s chemical behavior, as they are the electrons involved in chemical bonding.
Meet Beryllium’s Valence Electrons
In the case of beryllium, the atomic number of 4 implies the presence of 4 electrons. Two of these electrons occupy the innermost shell, leaving 2 valence electrons eagerly awaiting interaction. These valence electrons reside in the 2s orbital, poised to participate in the dance of chemical reactions.
Reactivity: A Tale of Valence Electrons
Beryllium’s low number of valence electrons has a profound impact on its reactivity. It readily forms ionic bonds with other elements, a testament to its eagerness to shed its valence electrons. This tendency arises from the element’s desire to achieve a stable electron configuration, a state where its outermost electron shell mirrors that of the noble gas helium.
Beryllium’s 2 valence electrons in the outermost 2s orbital are the conductors of its chemical symphony. They determine the element’s reactivity, shaping its ability to form ionic bonds and interact with other elements. Understanding valence electrons is the key to unlocking the secrets of beryllium’s chemistry.
Understanding Valence Electrons: The Key to Beryllium’s Chemistry
In chemistry, valence electrons play a pivotal role in determining the reactivity and properties of elements. These are the electrons that occupy the outermost energy level, or valence shell, of an atom. They are responsible for the chemical bonds that atoms form with each other.
Imagine atoms as tiny solar systems, with a nucleus at the center and electrons orbiting it like planets. The outermost electrons are the most loosely bound and can easily be shared or exchanged, forming the basis of chemical reactions.
In the case of beryllium, its atomic number of 4 indicates that it has 4 electrons. Two of these electrons fill the inner energy level, while the remaining two reside in the valence shell. These valence electrons make beryllium highly reactive, as it seeks to complete its valence shell by gaining or sharing electrons with other atoms.
Valence Electrons in Beryllium
- Highlight that the atomic number of beryllium (4) indicates the presence of 4 electrons.
- Explain that beryllium has 2 valence electrons, located in the outermost 2s orbital.
Valence Electrons in Beryllium: Unlocking the Chemistry of a Remarkable Element
In the realm of chemistry, valence electrons play a crucial role in shaping the properties and reactivity of elements. These electrons, residing in the outermost shell of an atom, dictate the element’s ability to form bonds and interact with others. To explore the significance of valence electrons, let’s delve into the fascinating case of beryllium.
Beryllium, with an atomic number of 4, boasts a total of 4 electrons. These electrons are arranged in two electron shells: the inner 1s shell and the outer 2s shell. The electrons in the outermost shell, known as valence electrons, are responsible for beryllium’s chemical behavior.
Beryllium’s Valence Electrons: A Gateway to Bonding
Beryllium’s atomic number reveals the presence of 2 valence electrons, nestled in the 2s orbital. These electrons hold the key to beryllium’s bonding characteristics. Beryllium’s low number of valence electrons makes it a prime candidate for forming ionic bonds. In these bonds, beryllium readily loses its two valence electrons to achieve a stable electron configuration, resulting in a positively charged beryllium ion (Be2+).
Valence Electrons and Chemical Reactivity: A Tale of Two Atoms
The number of valence electrons an element possesses is a direct reflection of its reactivity. Elements with fewer valence electrons, like beryllium, tend to be more reactive than those with a full valence shell. This is because elements with fewer valence electrons have a greater tendency to lose or gain electrons to achieve a stable configuration.
In contrast, elements with a full valence shell are less reactive because they have no need to lose or gain electrons to stabilize their electron configuration. This difference in reactivity based on valence electrons explains the diverse chemical properties observed across the periodic table.
In the case of beryllium, its 2 valence electrons in the outermost 2s orbital are the driving force behind its chemical behavior. These electrons render beryllium prone to forming ionic bonds, making it a reactive element. Understanding the concept of valence electrons is essential for deciphering the chemistry of beryllium and countless other elements, unlocking the secrets of the molecular world.
Valence Electrons and the Reactivity of Beryllium
In the world of chemistry, electrons play a crucial role in determining the behavior and properties of elements. Valence electrons, in particular, hold the key to understanding the chemical reactivity of elements like beryllium. Let’s dive into the fascinating story of beryllium’s valence electrons and their impact on its reactivity.
Beryllium, an element with an atomic number of 4, possesses a unique electronic structure. Its two valence electrons reside in the outermost 2s orbital. These electrons, like restless explorers, eagerly seek out partners to form stable bonds. Due to beryllium’s low number of valence electrons, it exhibits a strong tendency to form ionic bonds.
Ionic bonds are formed when atoms transfer electrons to achieve a stable electron configuration. Beryllium, with its two valence electrons, readily donates them to other atoms, transforming into positively charged ions. This process allows beryllium to form stable compounds with elements that have a high electronegativity, such as oxygen and fluorine.
The importance of valence electrons extends beyond just ionic bond formation. These electrons also dictate the chemical properties and reactivity of beryllium. Elements with a high number of valence electrons tend to be more reactive, while those with a low number, like beryllium, are typically less reactive. This is because the fewer valence electrons an element has, the more tightly they are held, making it more difficult for them to participate in chemical reactions.
In the case of beryllium, its two valence electrons are held securely, making it relatively unreactive. However, under specific conditions, beryllium can still form bonds, albeit with a limited range of elements. Its reactivity is influenced by factors such as temperature, pressure, and the presence of other reactive substances.
Understanding the valence electrons of beryllium provides a glimpse into its chemical behavior and reactivity. These electrons play a pivotal role in shaping beryllium’s interactions with other elements, ultimately determining its properties and applications in various fields of science and engineering.