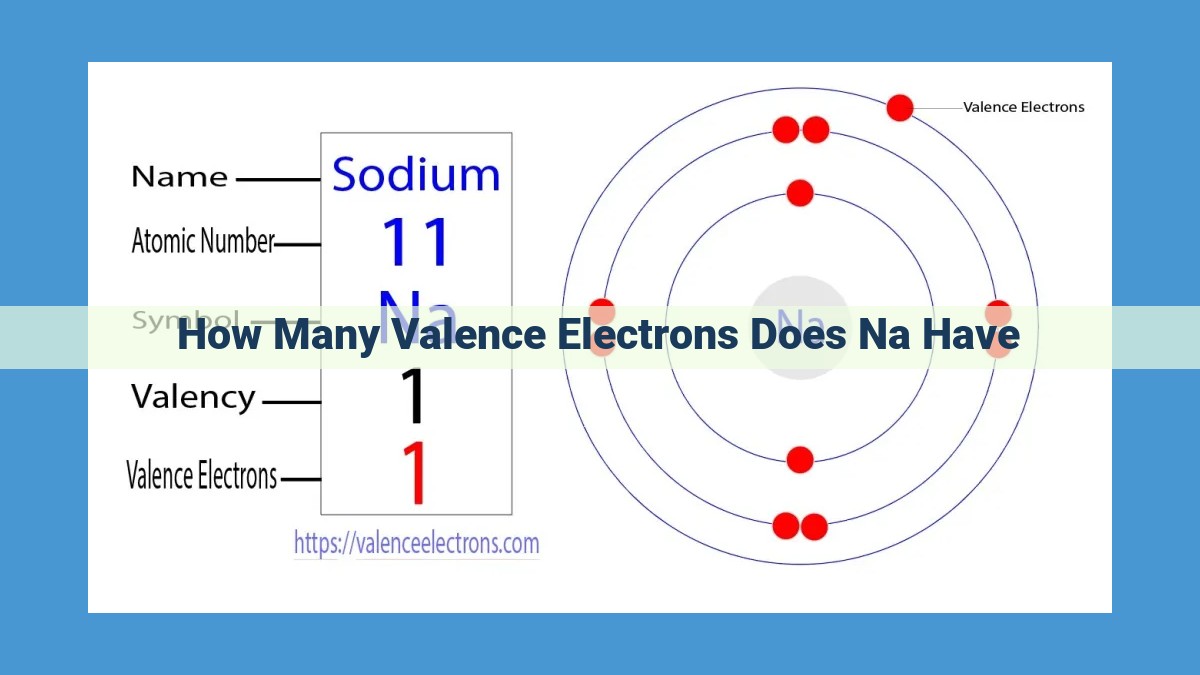
Valence electrons, located in an atom’s outermost orbital, play a crucial role in determining chemical bonding and reactivity. Sodium (Na), an alkali metal, has one valence electron. This is because its atomic number (11) indicates that it has 11 electrons in a neutral state, with one in the valence shell. Elements in the same group (Group 1) share the same number of valence electrons. Na’s single valence electron makes it highly reactive, driving its interactions with other elements to achieve stable electron configurations through bonding, oxidation-reduction reactions, and influence on electronegativity and ionization energy.
Valence Electrons: The Key Players in Chemical Bonding and Reactivity
In the realm of chemistry, valence electrons hold a pivotal position, influencing the behavior of elements and determining their ability to form bonds. These are the electrons found in the outermost orbital of an atom and play a crucial role in chemical reactions. Let’s embark on a journey to understand the significance of valence electrons, using sodium (Na), a highly reactive alkali metal, as an example.
Understanding Valence Electrons
Every element has a unique atomic number, which corresponds to the number of protons in its nucleus. This atomic number also determines the number of electrons in a neutral atom. Atoms strive for stability by achieving a full outermost electron shell, and valence electrons are the key to unlocking this stability.
Understanding Valence Electrons
As we journey through the fascinating world of chemistry, let’s unravel the secrets of valence electrons, the gatekeepers to chemical bonding and reactivity.
Picture an atom as a miniature solar system, with a nucleus at its center and electrons orbiting around it like planets. The atomic number of an element tells us the number of positively charged protons in the nucleus. This number also corresponds to the number of electrons in a neutral atom.
Electrons, however, prefer to hang out in specific regions around the nucleus, forming concentric circles called shells. Each shell can hold a certain number of electrons. The outermost shell, the one most distant from the nucleus, is the most important for us. It’s here that we find the valence electrons, the stars of our chemical story.
Valence electrons are like the social butterflies of the atomic world. They’re the ones that interact with other atoms, forming bonds and determining the reactivity of the element. The number of valence electrons an atom has is determined by its position on the Periodic Table. Elements in the same group, the vertical columns, have the same number of valence electrons.
Take sodium (Na), for example. It’s an alkali metal that sits in Group 1 of the Periodic Table. This means sodium has just one valence electron, making it a highly reactive element. Sodium’s single valence electron is eager to break free and form bonds with other atoms, giving it a strong tendency to react.
Understanding Valence Electrons in Sodium (Na)
In the realm of chemistry, valence electrons hold the key to understanding the behavior and reactivity of elements. These electrons reside in the outermost orbital of an atom, eager to engage in chemical bonding, the dance that forms molecules.
Sodium (Na), an alkali metal, is a prime example of how valence electrons shape an element’s nature. Sodium’s atomic number, 11, reveals the total number of electrons in a neutral atom. Electron configuration, a map of these electrons’ arrangement, shows sodium with one electron in its outermost orbital. This lone valence electron is the driving force behind sodium’s high reactivity.
Elements belonging to the same group in the periodic table share a common characteristic: they have the same number of valence electrons. Sodium resides in Group 1, also known as the alkali metals, meaning it has one valence electron. This shared trait among alkali metals grants them similar chemical properties, making them highly reactive and prone to forming positive ions (cations).
Understanding the Significance of Valence Electrons
Understanding valence electrons is crucial, as they play a vital role in determining the chemical properties of an element. Valence electrons are the electrons in the outermost energy level of an atom, which determine its chemical reactivity.
Sodium: A Case Study
Let’s take sodium (Na) as an example. Sodium is a highly reactive alkali metal with a single valence electron. To understand its reactivity, we need to delve into the concept of valence electrons.
Atomic Structure and Valence Electrons
Atomic number refers to the number of protons in an atom’s nucleus. It also determines the number of electrons in a neutral atom. The arrangement of electrons in different energy levels is known as electron configuration. Valence electrons are located in the outermost energy level and dictate an atom’s chemical behavior.
Valence Electrons in Sodium
Sodium belongs to Group 1 of the periodic table, known as alkali metals. Elements in the same group have the same number of valence electrons. Sodium has one valence electron, located in the outermost energy level.
Chemical Bonding and Valence Electrons
Valence electrons play a critical role in chemical bonding. To achieve a stable electron configuration, atoms strive to have a full valence shell. They can do this by forming bonds with other atoms, sharing or transferring valence electrons.
Ionization Energy and Valence Electrons
Ionization energy is the energy required to remove an electron from an atom. Elements with fewer valence electrons generally have lower ionization energies. Sodium, with its single valence electron, has a relatively low ionization energy, making it easy to remove the electron.
Electronegativity and Valence Electrons
Electronegativity measures an atom’s ability to attract electrons. Elements with higher electronegativity values have a greater ability to attract electrons. Valence electrons influence electronegativity, as they determine the number of electrons an atom can share or accept.
Oxidation States and Valence Electrons
Oxidation states represent the possible charges an element can exhibit in chemical compounds. Valence electrons determine the oxidation states an element can adopt. Sodium, with its single valence electron, can exhibit an oxidation state of +1.